

Our laboratory studies breast cancer, with the main goal of understanding the mechanisms underlying breast cancer invasion and metastasis so as to develop effective treatments.
Employing pathology, cell and molecular biology, and genetics our laboratory discovered several major events associated with metastasizing breast cancers, which are the basis of our lab investigations. Our main ongoing projects are the following.
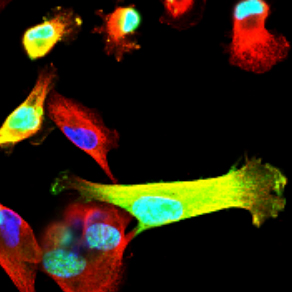
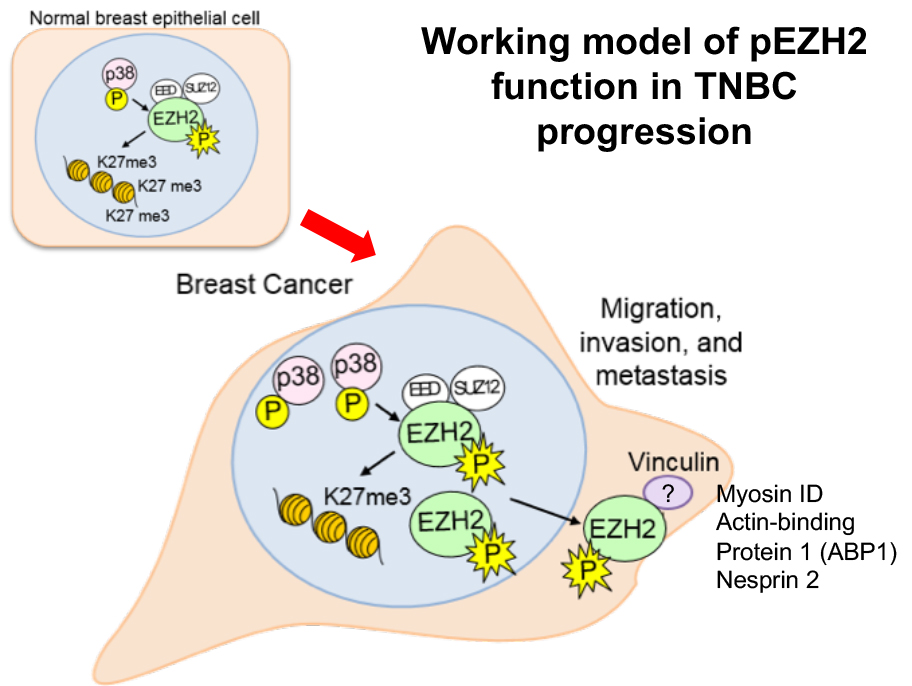
Understanding the mechanisms by which EZH2 promotes breast cancer metastasis.
Studies of EZH2 in our lab span over a decade. Our early studies in 2003, discovered a mechanistic link between the cellular transcriptional memory regulators and breast cancer. We identified that the Polycomb Group protein Enhancer of zeste 2 (EZH2) is upregulated in invasive carcinomas and that EZH2 overexpression induces breast cancer cell invasion and growth. Our subsequent investigations showed that EZH2 is predominantly expressed in ER-negative invasive carcinomas and provided in vivo evidence of the oncogenic role of EZH2 in the breast, by developing a unique transgenic mouse model of mammary-specific EZH2 overexpression. Studies towards elucidating the mechanism of EZH2 oncogenic functions resulted in the novel observation that in ER-negative carcinomas, EZH2 has cytoplasmic functions. Specifically, p38 induced EZH2 phosphorylated at T367 induces its cytoplasmic localization and allows binding of EZH2 to cytoskeletal proteins that trigger migration and invasion. We are currently investigating these mechanisms further using specific deletion mutants, patient-derived cells, as well as animal models.
—
Read our latest paper: www.nature.com
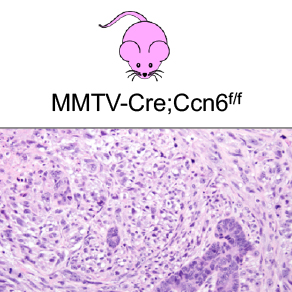
Investigation of the tumor suppressor function of CCN6, a matrix-associated protein, in breast cancer.
CCN6 is a matricellular protein with functional, rather than structural roles in the extracellular microenvironment. We showed that secreted CCN6 binds to growth factors including insulin-like growth factor 1 (IGF1) and bone morphogenetic protein 4 (BMP4) to reduce their growth and invasion promoting effects on breast cancer cells. Our work identified that loss of CCN6 in breast epithelial cells leads to an epithelial to mesenchymal transition by regulating Snai1, Snai2, and ZEB1 transcription factors, and promotes growth factor independent growth of breast epithelial cells. A major breakthrough in our lab has been the recent discovery that CCN6 is a tumor suppressor of spindle metaplastic breast carcinomas through the generation and characterization of MMTV-Cre;Ccn6fl/fl mice, and that CCN6 protein is reduced or lost in 90% of human spindle metaplastic carcinoma tissue samples. The current work aims to characterize this model and understand the mechanisms of CCN6 tumor suppression in detail.
—
Read Press Release: labblog.uofmhealth.org/
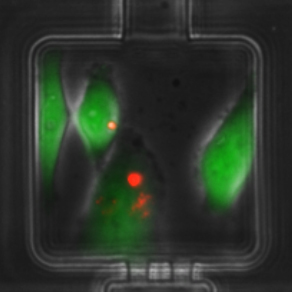
Elucidating the role and mechanism of mesenchymal stem/stromal cells (MSCs) in the tumor microenvironment on breast cancer progression.
This project stems from our observation that in co-cultures of breast cancer cells with MSCs, there is an emergence of a hybrid cell population. This led to mechanistic studies demonstrating that breast cancer cells have the ability to engulf (or cannibalize) MSCs, which induces heritable transcriptomic changes in breast cancer cells and triggers a metastatic phenotype. Using histopathology work, we detected hybrid multinucleated cells in breast cancer distant metastasis. This work led to collaborations with researchers from the College of Engineering and led to the design of a microfluidic cell pairing device that allows visualization and quantification of MSC engulfment by human breast cancer cells, and laser-retrieval of single-cell populations without damage.
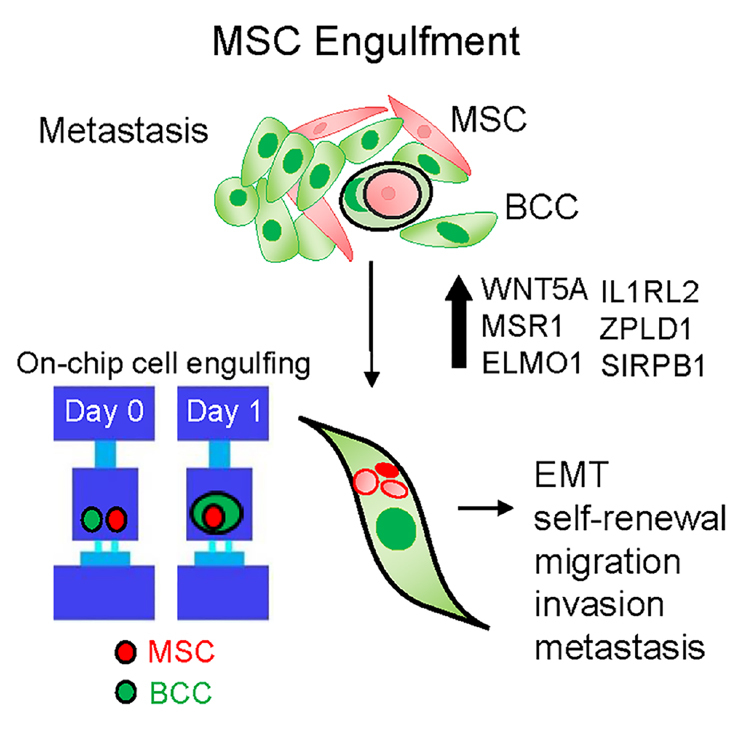
—
Read the press release: labblog.uofmhealth.org/
Utilization of state of the art transcriptome analyses and quantitative proteomics, and proteogenomics to understand defining molecular alterations in breast cancer.
As a practicing breast pathologist, I am cognizant of the unmet needs in clinical practice. One of those needs is the understanding of the pathobiology of aggressive forms of breast cancer, such as metaplastic and inflammatory breast cancer, and the discovery and validation of useful biomarkers that can inform the biologic tumor behavior and response to chemotherapy. To this end, we have recently completed comprehensive quantitative proteomics and proteogenomics analysis of human metaplastic carcinomas. These studies have shed light on novel targets that we are investigating at this time.
—
Read our paper at: www.ncbi.nlm.nih.gov/
 ON THE COVER
ON THE COVER
Breast team reviewing a patient's slide. (From left to right) Ghassan Allo, Fellow; Laura Walters, Clinical Lecturer; Celina Kleer, Professor. See Article 2014Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Autopsy Technician draws blood while working in the Wayne County morgue. See Article 2016Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Sriram Venneti, MD, PhD and Postdoctoral Fellow, Chan Chung, PhD investigate pediatric brain cancer. See Article 2017Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Director of the Neuropathology Fellowship, Dr. Sandra Camelo-Piragua serves on the Patient and Family Advisory Council. 2018Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Residents Ashley Bradt (left) and William Perry work at a multi-headed scope in our new facility. 2019Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Kristine Konopka (right) instructing residents while using a multi-headed microscope. 2020Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Patient specimens poised for COVID-19 PCR testing. 2021Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Pantanowitz demonstrates using machine learning in analyzing slides. 2022Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
(Left to Right) Drs. Angela Wu, Laura Lamps, and Maria Westerhoff. 2023Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|

MLabs, established in 1985, functions as a portal to provide pathologists, hospitals. and other reference laboratories access to the faculty, staff and laboratories of the University of Michigan Health System’s Department of Pathology. MLabs is a recognized leader for advanced molecular diagnostic testing, helpful consultants and exceptional customer service.