

Technological advances have contributed significantly to improved detection rates of important microbial pathogens. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry, historically used in only in clinical chemistry laboratories, is now improving diagnostic speed and accuracy in clinical microbiology laboratories.
The primary goal of the Clinical Microbiology Laboratory is to identify causative agents of infectious diseases in patient specimens. Traditionally, this is achieved by first isolating an organism in culture and using a variety of different physical and chemical features of the organism to identify it. However, the identification procedures can be time-consuming and cumbersome, often requiring days before the final result is available. MALDI-TOF mass spectrometry has recently emerged as an important, rapid and accurate tool for organism identification, providing results in just a few minutes.
Once an organism is isolated from a clinical specimen, a small amount is spotted on a stainless steel target plate, mixed with a chemical matrix and allowed to dry. When placed into the MALDI-TOF instrument, the spot on the target plate is subjected to laser pulses. The now desorbed ionized molecules from the isolate are accelerated through a flight tube by an electromagnetic field. The TOF of the analytes to the detector is then measured and produces a characteristic spectrum from which an organism can be identified. This is accomplished by comparing the resultant spectrum to a database containing spectral patterns of various clinically-relevant organisms. The software then matches the spectrum in question, providing an identification.
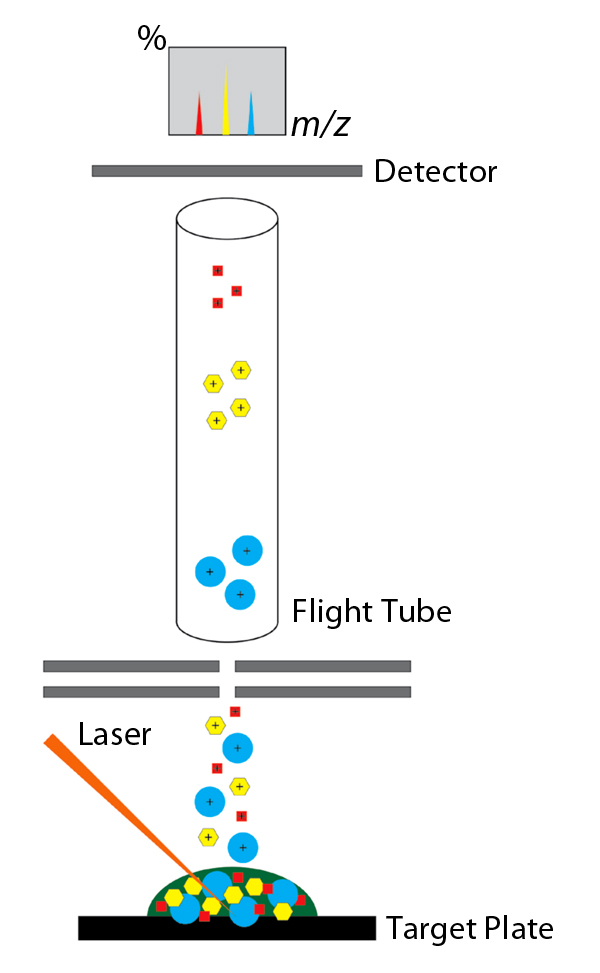
Diagram of process. MALDI-TOF mass spectrometry is suitable for the microbiology laboratories because samples require minimal preparation and identifications are made in less than one minute. MALDI-TOF also provides excellent specificity, low operating costs, and requires minimal maintenance. The identification database is continuously updated with clinically-relevant organisms to improve the diagnostic capacity of the system.
Although this technology is powerful, there are a few limitations. The database is not entirely complete, so not all organisms are able to be identified (<5% unidentified). In addition, the acquisition costs of the instrument are high; but because of the extremely low operating costs, and the high volume of samples that can be tested on the system, the return on investment can be relatively quick.
In collaboration with our institutional Antimicrobial Stewardship Team (AST), we have recently published a study* demonstrating that rapid identification of isolates using MALDI-TOF yields clinically-significant benefits to our patients and health system overall when coupled with active AST interventions. Not only were microbiology reporting times reduced, but patients whose cultures were identified using MALDI-TOF had significantly shorter times to placement on optimal antimicrobial therapy, reduced length-of-stay, and reduced mortality compared to patients whose cultures were identified using conventional methods.
This recent implementation of MALDI-TOF in the Clinical Microbiology Laboratory has clearly demonstrated positive clinical and operational impacts, and is serving as a template for the continued incorporation of the latest technology in our clinical microbiology laboratory.
*Huang AM, Newton DW, Kunapuli A, Gandhi TN, Washer LL, Isip J, Collins CD, Nagel JL. 2013. Rapid Organism Identification via Matrix-Assisted Laser Desorption Ionization Time-of-Flight Combined with Antimicrobial Stewardship Team Intervention Decreases Mortality and Improves Time to Clinical Response in Adult Patients with Bacteremia and Candidemia. Clinical Infectious Diseases. 57:1237-45.
 ON THE COVER
ON THE COVER
Breast team reviewing a patient's slide. (From left to right) Ghassan Allo, Fellow; Laura Walters, Clinical Lecturer; Celina Kleer, Professor. See Article 2014Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Autopsy Technician draws blood while working in the Wayne County morgue. See Article 2016Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Sriram Venneti, MD, PhD and Postdoctoral Fellow, Chan Chung, PhD investigate pediatric brain cancer. See Article 2017Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Director of the Neuropathology Fellowship, Dr. Sandra Camelo-Piragua serves on the Patient and Family Advisory Council. 2018Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Residents Ashley Bradt (left) and William Perry work at a multi-headed scope in our new facility. 2019Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Kristine Konopka (right) instructing residents while using a multi-headed microscope. 2020Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Patient specimens poised for COVID-19 PCR testing. 2021Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Pantanowitz demonstrates using machine learning in analyzing slides. 2022Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
(Left to Right) Drs. Angela Wu, Laura Lamps, and Maria Westerhoff. 2023Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Illustration representing the various machines and processing used within our labs. 2024Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Rendering of the D. Dan and Betty Khn Health Care Pavilion. Credit: HOK 2025Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|

MLabs, established in 1985, functions as a portal to provide pathologists, hospitals. and other reference laboratories access to the faculty, staff and laboratories of the University of Michigan Health System’s Department of Pathology. MLabs is a recognized leader for advanced molecular diagnostic testing, helpful consultants and exceptional customer service.