Study by Russell Ryan and Colleagues Links Mutations in Notch Gene to Role in B Cell Cancers
By Elizabeth Walker | October 24 2017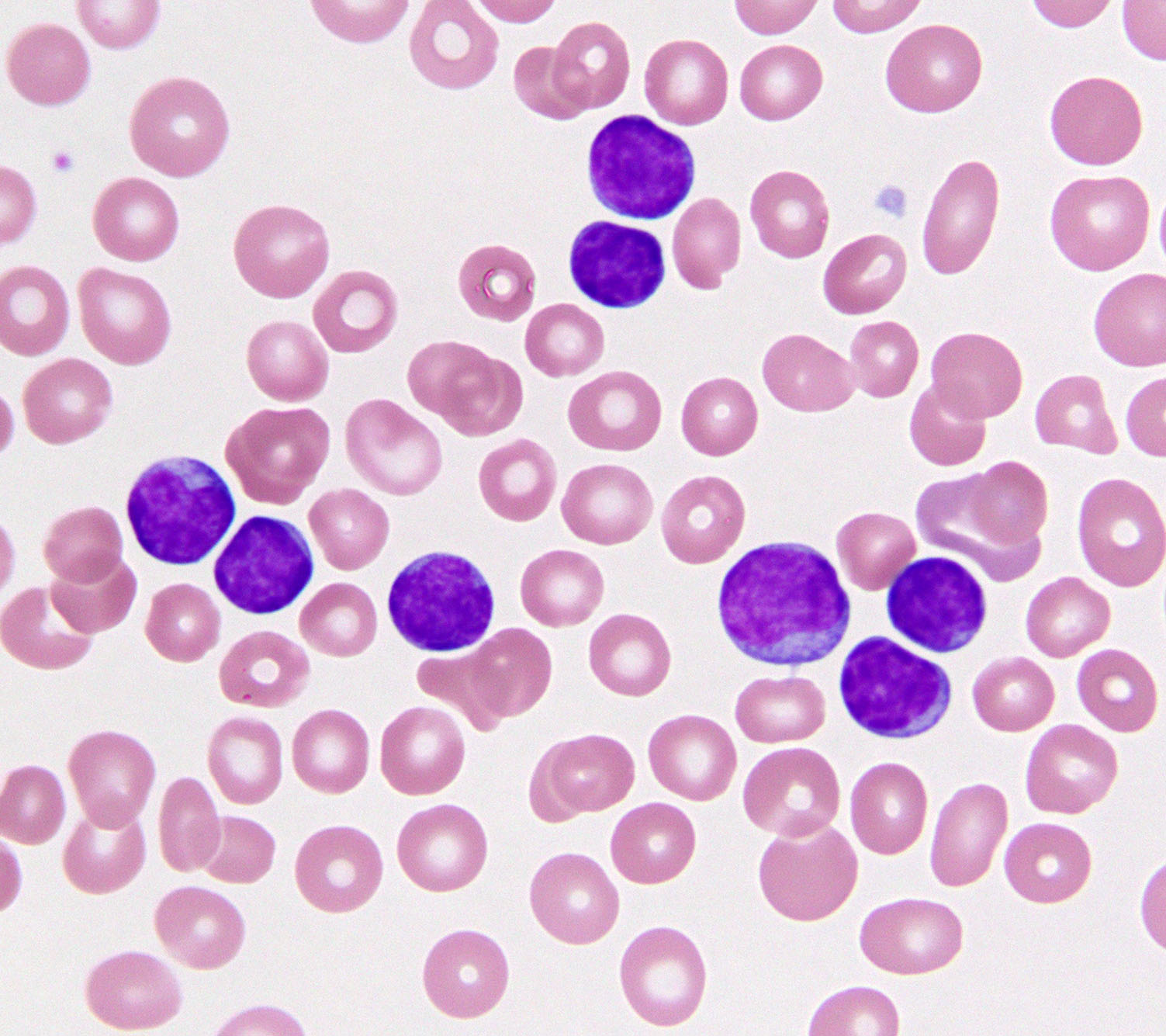 An image of chronic lymphocytic leukemia cells in the peripheral blood. Photo credit - Elizabeth Morgan, MD Notch is one of the most frequently mutated genes in chronic lymphocytic leukemia (CLL), the most common leukemia in adults in the United States. It is also often mutated in other common B cell tumors, such as mantle cell lymphoma. However, the role of Notch in these cancers has been uncertain. Now, work done by Michigan Medicine’s Russell Ryan, MD, Assistant Professor of Pathology, and colleagues at Massachusetts General Hospital, Brigham and Women’s Hospital, and the University of Pennsylvania, provides new insights into how Notch drives the growth of B-cell cancers. The teams report their findings in Cell Reports.
An image of chronic lymphocytic leukemia cells in the peripheral blood. Photo credit - Elizabeth Morgan, MD Notch is one of the most frequently mutated genes in chronic lymphocytic leukemia (CLL), the most common leukemia in adults in the United States. It is also often mutated in other common B cell tumors, such as mantle cell lymphoma. However, the role of Notch in these cancers has been uncertain. Now, work done by Michigan Medicine’s Russell Ryan, MD, Assistant Professor of Pathology, and colleagues at Massachusetts General Hospital, Brigham and Women’s Hospital, and the University of Pennsylvania, provides new insights into how Notch drives the growth of B-cell cancers. The teams report their findings in Cell Reports.
The researchers found that in B cell tumors, mutated overactive versions of the Notch protein directly drive the expression of the Myc gene and many other genes that participate in B cell signaling pathways. Myc is a critical gene in governing cell proliferation and survival, activities that it carries out by regulating the expression of other genes involved in cell metabolism.
B cell signaling pathways are the current targets of several therapies used to treat B cell malignancies such as CLL. “An important translational implication of this research is that we hope that by combining Notch inhibitors with drugs that target B-cell signaling we can better treat these B-cell cancers,” said senior author Warren Pear, MD, PhD, a professor of Pathology and Laboratory Medicine at Penn Medicine. “Although this is true of many transcription factors, it has been difficult to develop therapeutics that directly target the Myc protein, an alternative approach may be to target the proteins that regulate Myc expression.” Notably, multiple Notch inhibitors are in various stages of clinical development as potential cancer therapies.
The mechanism used by Notch to regulate Myc in B cells is distinct from the mechanism used in other cell types, such as T cells, where Notch also regulates Myc. The team found that Notch uses different regulatory switches in the genome, called enhancers, in different cell types. This raises the issue of why evolution would select for this complexity. One reason may be that Myc needs to be under very tight control in each cell. For example, in the mouse model of Notch-induced T-cell leukemia, the Penn group previously found that the difference between inducing a T cell tumor or not is a doubling of Myc transcription by Notch. As Notch appears to use cell type-specific machinery to regulate Myc, it may be possible to target the Notch-Myc signaling path in a way that does not disrupt this path in other cell types.
“Another surprising finding was the direct link between Notch and genes involved in other B cell signaling pathways,” said study co-first author Russell Ryan, MD, who is continuing his work on Notch and lymphoma in his new laboratory at the University of Michigan. “For example, Notch activates genes involved in B cell receptor signaling, which is an established drug target in these B cell cancers. The challenge now will be to understand what this might mean for treatment of patients with Notch-activated B-cell leukemias and lymphomas.” The team plans to test the synergy between Notch and B-cell signaling inhibitors. If they find a relationship, the next step would be to stimulate interest in a clinical trial.
The study was a collaboration between the laboratories of Warren Pear (University of Pennsylvania), Bradley Bernstein (Massachusetts General Hospital) and Jon Aster (Brigham and Women’s Hospital). Lead authors of the study were Russell Ryan (formerly of the Bernstein laboratory, now Assistant Professor of Pathology at the University of Michigan) and Jelena Petrovic (of the Pear laboratory).
This work was funded in part by the National Institutes of Health (P01 CA119070, R01AI047833, U01HL100405, K08CA208013), the Leukemia and Lymphoma Society, the Penn Epigenetics Institute, the Loring fund for Lymphoma Research, and donors to the MGH Center for Lymphoma: Future Discovery Project.
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
