Identifying an “Achilles heel” of an aggressive subset of acute leukemias
By Anastazia Hartman | February 17 2023Hsiang-Yu (David) Hu, a Molecular and Cellular Pathology graduate student in Dr. Andrew Muntean’s lab, recently published his first first-authored article, The ENL YEATS epigenetic reader domain critically links MLL-ENL to leukemic stem cell frequency in t(11;19) Leukemia, in Leukemia.
Originally from Taiwan, David received his undergraduate degree from the University of Minnesota, then traveled to Michigan to complete a master of science in bioinformatics and a doctorate in molecular and cellular pathology. David joined Dr. Andrew Muntean’s lab in 2018 where he has been studying epigenetics in acute myeloid leukemia.
 “Ever since high school, I knew I was interested in cancer therapeutics. Originally, I was thinking more about going to pharmacy school or medical school, but after spending a couple semesters as an undergraduate research assistant, the translational side of research got me interested in studying the mechanisms behind tumorigenesis” David said. “I was initially attracted to the MCP program because of the diverse research done here on different kinds of diseases, including multiple different types of cancers.”
“Ever since high school, I knew I was interested in cancer therapeutics. Originally, I was thinking more about going to pharmacy school or medical school, but after spending a couple semesters as an undergraduate research assistant, the translational side of research got me interested in studying the mechanisms behind tumorigenesis” David said. “I was initially attracted to the MCP program because of the diverse research done here on different kinds of diseases, including multiple different types of cancers.”
In the Muntean lab, David’s research is focused on an epigenetic reader domain called the YEATS domain. He is working to identify its function in relation to a rare and aggressive subset of acute leukemia driven by the MLL-ENL fusion protein.
“Our lab is committed to understanding how epigenetics contributes to leukemia initiation and progression to identify more effective therapeutic targets,” said Dr. Muntean. “Our studies, spearheaded by David Hu, Nirmalya Saha, Ph.D,. and others in our group, found a subset of leukemia patients invariably retain an epigenetic reading domain in MLL-ENL fusion proteins that drive leukemia. Importantly, the presence of the epigenetic reader domain was linked with preserving leukemia stem cells making it an ideal target for further therapeutic development.”
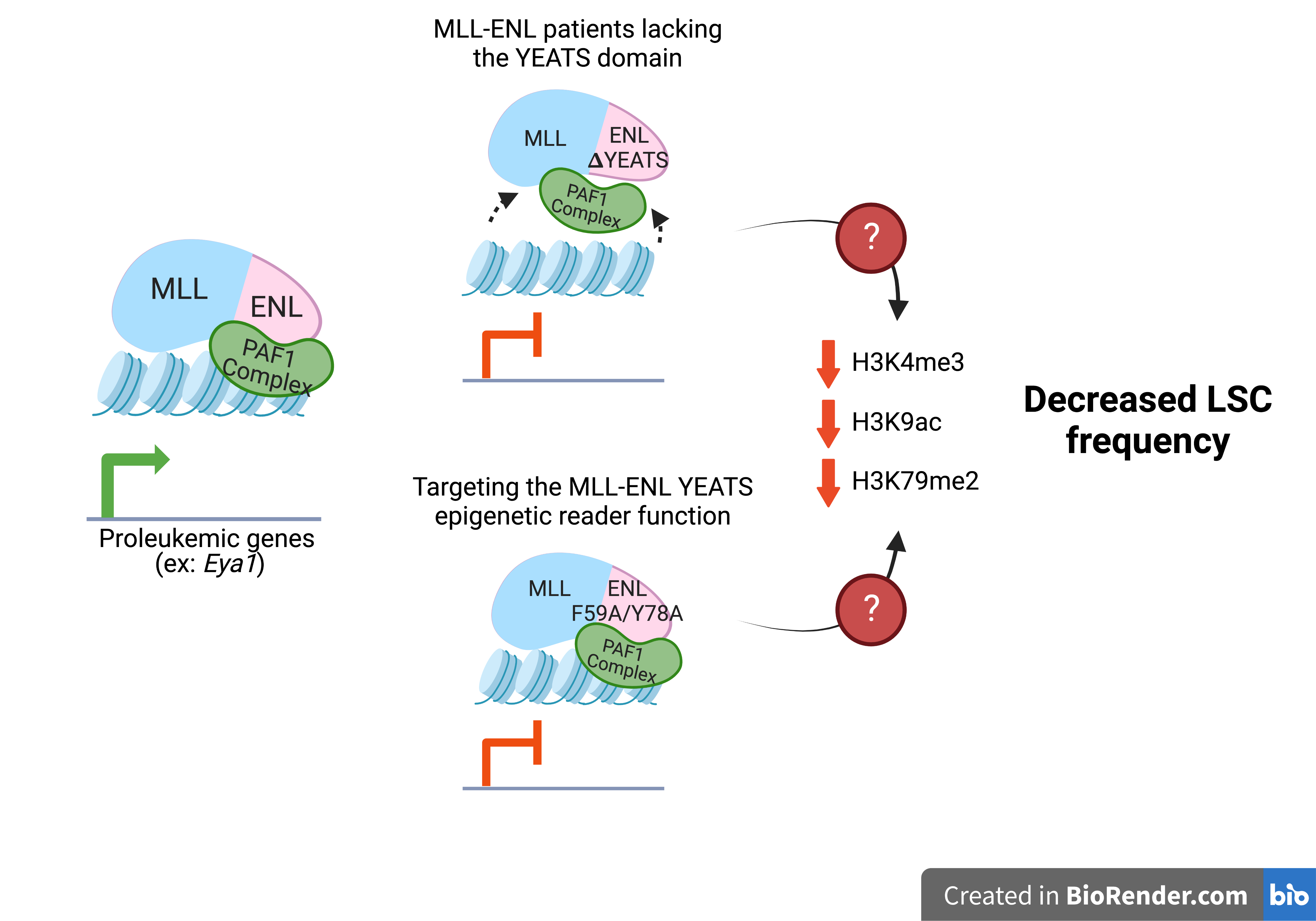 “Our model shows that this epigenetic reader domain on the fusion protein is linked to MLL-ENL leukemia disease outcome and is especially important for the regulation of a subset of MLL-ENL target genes, both transcriptionally and epigenetically,” added David. “Having these mechanistic insights are going to help us predict how MLL-ENL patients might respond to YEATS inhibitor.”
“Our model shows that this epigenetic reader domain on the fusion protein is linked to MLL-ENL leukemia disease outcome and is especially important for the regulation of a subset of MLL-ENL target genes, both transcriptionally and epigenetically,” added David. “Having these mechanistic insights are going to help us predict how MLL-ENL patients might respond to YEATS inhibitor.”
As David and his colleagues learned more about the YEATS domain, it was time to share their findings in David’s article, The ENL YEATS epigenetic reader domain critically links MLL-ENL to leukemic stem cell frequency in t(11;19) Leukemia. “This is my first first-author publication. So, if I were to describe it…It was a great learning opportunity because every step along the process was new to me,” David said. “From the initial process of data collection, manuscript assembly, to submission, getting and addressing reviewers’ feedback... it was all-around a labor-intensive process, but extremely rewarding at the end.”
While David’s publication has been an exciting experience, he knows that it couldn’t have been done without the help of his PI, Dr. Muntean, his co-first author, Dr. Saha Nirmalya, and many other collaborators. In the future, David hopes that follow-up research will further the development of therapeutics to treat MLL-ENL Leukemia patients.
Citation:
Hu H, Saha N, Yang Y, Ahmad E, Lachowski L, Shrestha U, Premkumar V, Ropa J, Chen L, Teahan B, Grigsby S, Marschalek R, Nikolovska-Coleska Z, Muntean AG. The ENL YEATS epigenetic reader domain critically links MLL-ENL to leukemic stem cell frequency in t(11;19) Leukemia. Leukemia. 2023 Jan;37(1):190-201. doi: 10.1038/s41375-022-01765-0. Epub 2022 Nov 26. PMID: 36435883.
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
