

What is it?
It's a departmental-wide, comprehensive project to ensure the Pathology Department can efficiently manage all specimens through the stages of pre-analytic, analytic, and post-analytic from multiple sources to multiple lab locations.
Why are we doing this?
During strategic meetings in January 2016, leaders from Clinical Pathology, Anatomic Pathology, and Pathology Informatics all expressed interest in beginning a formal approach to improving our management of specimens. Particularly concerning is the number of specimens that are missing or lost and the impact this can have on patients and families. With the pending move of many of the labs to NCRC in 2018, a more formalized tracking of specimens was recognized as a need. Lastly, we are required by the College of American Pathologists (CAP) under GEN.40530 to "For specimens submitted to the laboratory from remote sites, there is a tracking system and record to ensure that all specimens are actually received."
Where do we start?
While the Patient Asset Management Initiative (PAMI) will focus on the entire process indicated below, the Steering Committee has recommended we begin with Specimen Tracking, as designated with the area outlined below. This will enable Pathology to implement necessary changes within the department, an area we can readily control. After some early successes with this, an investigation about improvements upstream of Pathology will be investigated with our partners within the health system.
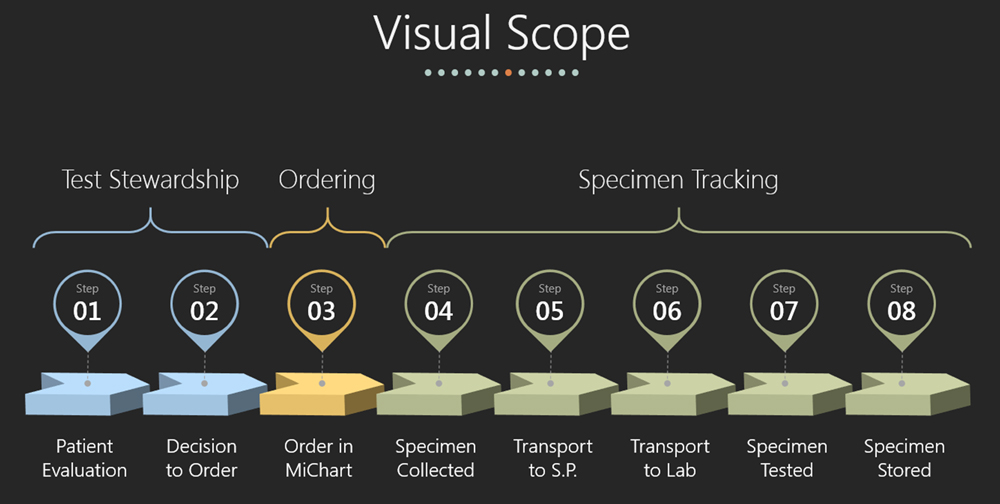
What is the scope of specimen tracking?
To design and implement a tracking system that begins when a specimen is physically collected through delivery, testing, and disposition/storage.
While creating solutions, what are the guiding principles?
The steering committee has outlined the following 2 guiding principles:
What constitutes a specimen?
The definition of a specimen from College of American Pathologists:
Primary specimen - The body fluid, tissue, or sample submitted for examination, study or analysis. It may be within a collection tube, cup, syringe, swab, slide, data file, or another form as received by the laboratory.
Secondary specimen - Any derivative of the primary specimen used in subsequent phases of testing. It may be an aliquot, dilution tube, slide, block, culture plate, reaction unit, data extract file, image, or another form during the processing or testing of a specimen.
Where did we start?
The best place to start with all projects is to clearly understand the current state. Through documentation sessions called "Power Hours" we met with lab representatives (techs and managers) to outline the current process with respect to specimen tracking. These documents are a type of value stream map (VSM).
What's the goal during these Power Hours?
By mapping the current processes, we were able to identify potential gaps or risks in the system. These gaps were evaluated across all labs and grouped according to similarity. This has provided opportunities for the labs to work together in order to create solutions to the potential risks.
Now that we have the different gaps identified, what will we do?
With input from the steering committee, we identified which gaps should have formal projects created to address and prioritize the activity for each project. The areas of focus that were selected are indicated below.
Who will be helping with these projects?
You! Project teams will be formed across the labs to help determine the most appropriate solution.
What are the first projects under PAMI?
There are a total of three projects that Pathology is first focusing on:
PathTrack: The technical design, construction, and implementation of a tracking system that incorporates information from Soft and enables users to track specimens throughout the life of a specimen.
Who should I contact if I have questions or I want to get more involved?
 ON THE COVER
ON THE COVER
Breast team reviewing a patient's slide. (From left to right) Ghassan Allo, Fellow; Laura Walters, Clinical Lecturer; Celina Kleer, Professor. See Article 2014Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Autopsy Technician draws blood while working in the Wayne County morgue. See Article 2016Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Sriram Venneti, MD, PhD and Postdoctoral Fellow, Chan Chung, PhD investigate pediatric brain cancer. See Article 2017Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Director of the Neuropathology Fellowship, Dr. Sandra Camelo-Piragua serves on the Patient and Family Advisory Council. 2018Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Residents Ashley Bradt (left) and William Perry work at a multi-headed scope in our new facility. 2019Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Kristine Konopka (right) instructing residents while using a multi-headed microscope. 2020Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Patient specimens poised for COVID-19 PCR testing. 2021Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Pantanowitz demonstrates using machine learning in analyzing slides. 2022Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
(Left to Right) Drs. Angela Wu, Laura Lamps, and Maria Westerhoff. 2023Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Illustration representing the various machines and processing used within our labs. 2024Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|

MLabs, established in 1985, functions as a portal to provide pathologists, hospitals. and other reference laboratories access to the faculty, staff and laboratories of the University of Michigan Health System’s Department of Pathology. MLabs is a recognized leader for advanced molecular diagnostic testing, helpful consultants and exceptional customer service.