Ferguson Lab

David O. Ferguson, MD, PhD
Associate Professor-Department of Pathology
Cancer Center Member-Division of Cancer Genetics
DNA REPAIR AND GENOMIC STABILITY
The Ferguson laboratory is interested in understanding how DNA repair in mammals works, and what roles it plays in cancer, immunity, and overall development. DNA damage occurs in many forms and numerous DNA repair complexes and pathways have evolved to combat them. People and experimental mice with inherited deficiencies in DNA repair may be prone to cancer at a very young age, may be immunocompromised, and can suffer from physical and developmental disabilities. We are focused on two specific types of DNA repair known to be involved in preventing cancer and other conditions; double strand break repair and the repair of disrupted DNA replication forks.
|
A |
B |
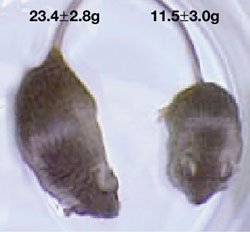
A. Two mice of the same age, but the one on the right lacks the XRCC4 DNA repair gene. It also lacks the P53 gene, and rapidly succumbs to cancer (from Gao, Ferguson et al, Nature 2000).
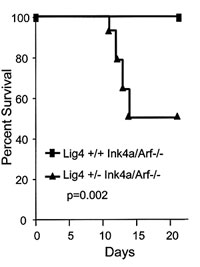
B. Mice lacking one copy of the Lig4 DNA repair gene die rapidly after exposure to gamma radiation (from Sharpless, Ferguson et al, Molecular Cell 2001.) |
DNA DOUBLE STRAND BREAK REPAIR
Failure to repair double strand breaks leads to a condition called genomic instability. As expected, this features fragmented chromosomes resulting from failure to repair DNA breaks. However, we and others have shown that deficient double strand break repair also leads to chromosomal translocations, which are comprised of material from two or more different chromosomes. This finding indicates that in the absence of one particular DNA repair pathway, other pathways may errantly repair breaks, leading to translocations. Cancer that develops in people and experimental mice with inherited double strand break repair defects results from this interplay between DNA repair pathways.
The laboratory is currently investigating how cells decide which pathway to use, and how pathways which normally prevent translocations, occasionally generate them. To this end we are generating and studying novel DNA repair deficient human cell lines and experimental mouse models. Among many avenues of investigation, we are utilizing an advanced technology known as Spectral Karyotyping (SKY) to understand the consequences of genomic instability in human and mouse models (see figure below)
|
A |
B |
C |
Spectral Karyotyping (SKY) of Mouse and human chromosomes
A) Random translocations arise in mouse fibroblasts deficient in members of the non-homologous end joining pathway (NHEJ) of double strand break repair (from Ferguson et al, PNAS 2000). B) Clonal translocations in tumors from mice deficient in both the DNA repair histone H2AX, and the cell cycle checkpoint protein p53 (from Bassing et al, Cell 2003.) C) This human tumor displays many of the characteristics found in DNA repair deficient mouse tumors. We are interested to learn if unknown DNA repair deficiencies are responsible for this type of genomic instability in spontaneous human tumors.
DNA REPAIR DURING REPLICATION
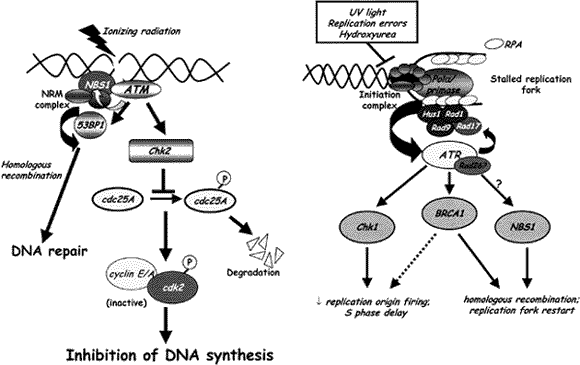
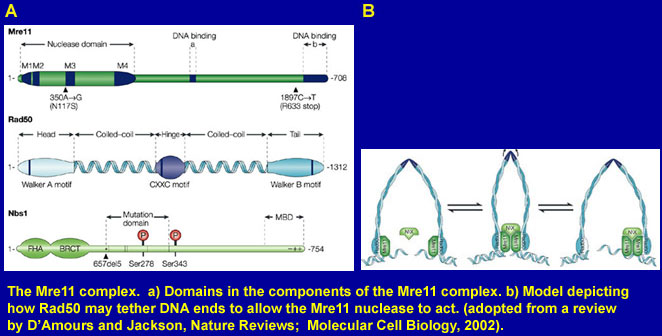
Proper replication of genomic DNA is essential for cellular survival, yet moving replication forks frequently encounter damaged or broken DNA that can stop replication. Cells employ several strategies to handle this challenge. First, an S phase checkpoint occurs which presumably slows replication while the offending lesions are dealt with. Second, some lesions are ignored by specialized DNA Polymerases that bypass them. Lastly, some lesions are repaired by specialized proteins. We are especially interested in a complex known as Mre11, which is composed of three proteins: Mre11, NBS, and Rad50. This complex lies at the intersection of all responses to DNA damage during S phase. It is involved in signaling to initiate the S phase checkpoint, and is directly involved in repair. This complex is crucial to survival, as mutant alleles that completely inactivate any member are lethal. It is also important in several aspects of human disease, as inherited mutant alleles that disable only some functions of the complex cause severe syndromes. For example, Nijmegen Breakage Syndrome results from mutation in the NBS gene and causes early predisposition to cancer, a defective immune system, and severe mental and physical handicaps. Mutations have been found in Mre11 that cause a similar, but milder syndrome known as ataxia telangiectasia like disorder (ATLD).
 |
|
Schematic of the relationship between DNA repair and DNA replication (adopted from a review b y Robert Abraham, Genes and Development, 2001).
|
In vitro biochemical studies indicate that Mre11 is a single strand DNA endonuclease and an exonuclease which may be held in proximity to DNA ends by interaction with Rad50. However, our understanding of the true functions of this complex in vivo has been hampered by the lethality of null alleles, which limit biological experiments. Through study of hypomorphic (partial loss of function) alleles of Mre11, we have identified important mutations that affect certain functions of the protein, but allow viability. Our studies of cells harboring these changes are allowing us to decipher the numerous functions of this complex in vivo. Engineered mice will teach us the roles of this complex in preventing cancer, sustaining the immune system and in other aspects of mammalian biology.
 |
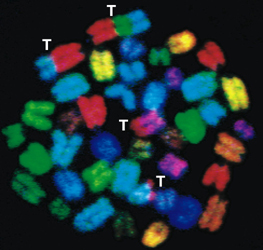
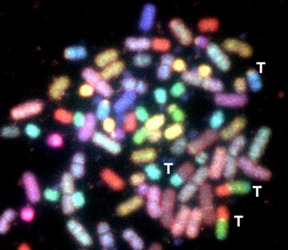
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
