

Differentiation, growth, and maintenance of epithelial cells are of fundamental importance to the normal develpment and physiology of all multi-cellular organisms. During embryonic develpment, epithelial tissues are formed by the growth and branchinng of existing epitelia or the posarization and conversion of mesenchyme into epithelia, both of which requirei inductive signalin. The lab has focused on cell-cell signaling mechanisms thta regulate the differentiation of epithelia and the patterning of complex tissues with both epithelial and mesenchymal components.
Recent work has idntified a new mouse gene that encodes a protein with homology to known regulators of the TGF-b superfamily of secreted signaling peptides. The protein is most homologous to Xenopus kielin, a dorsalizing factor during embryonic develpment. The closest Drosophila homologue is the crossveinless (cv-2) gene, which is thought to accentuate signaling in the develping wing disc. Thus, we have tentatively assigned the name KCP1 (Kielin/Cv2- Like Protein 1) to our new gene and its encoded protein. KCP1 is a large secreted protein with `9 repeated cysteine-rich domains, which are known to bind members of the TGF-b family directly. KCP1 is expressed in thee develping kidney at both early and late stages and corresponds to the formation of early epithelial structures within the intermediate mesoderm and to the formation of the proximinal tubules in the more developed metanephric kidney. The pattern of expression and the amino acid sequence suggest that KCP1 may regulate Activins, BMPs or TGF-bs signaling during develpment and maturation of renal tubules. Since TGF-bs and BMPs are known to regulate both epithelial differentiation and maturation in the kidney, how these signals are loalized and regulated with respet to receptor interactions is fundamental to understanding their bilogical function and poretial clinical applications.
Given the high degree of homology among signaling pathways across divergent species and the utility of Drosophila genetics, we will utilize both genetic and biochemical approaches in the mouse and fly. This cross-species approach enables us to address multiple aspects of KCP1 function and place it into biochemical and genetic pathways. The proposal addresses not only fundamental aspect s of develpmental biology but will provide new insights towards the role of TGF-b family signaling in differentiating and regenerating renal epithelial cells.
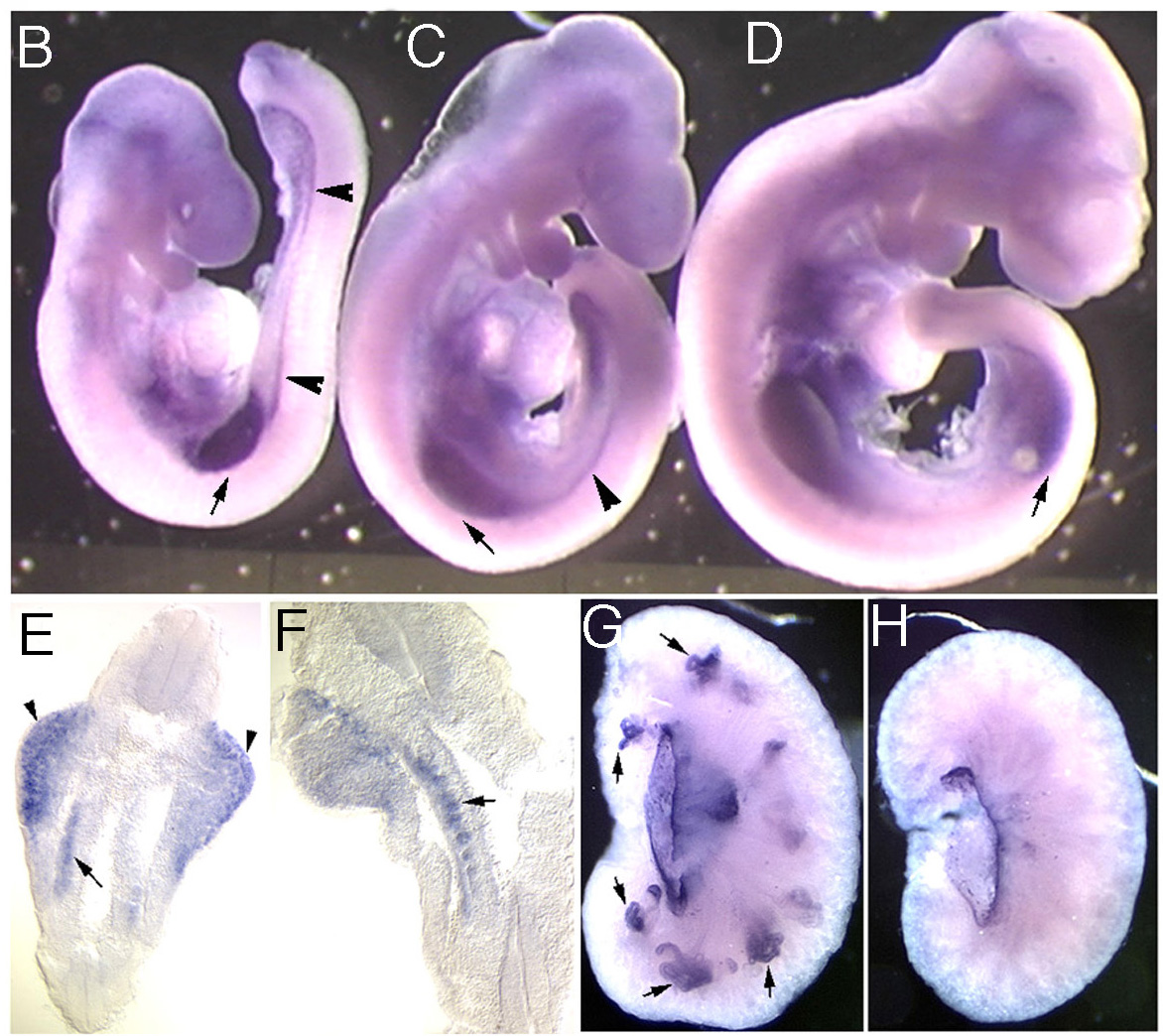
Figure 3 The expression of KCP1 in the develping mouse embryo is shown in the limb buds and in the intermediate mesoderm (B-F). The kidney is a derivative of this intermediate mesoderm and expresses KCP1 in develping proximal tubule progenitors at later stages (G,H).
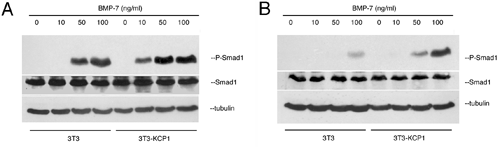
Figure 4 In a cell culture system, the KCP1 protein enhances signaling by BMP7. Activation of the BMP7 pathway results in Smad-1 phosphorylation which can be observed at lower doses in KCP1 expressing cells (A) and for longer times in B.
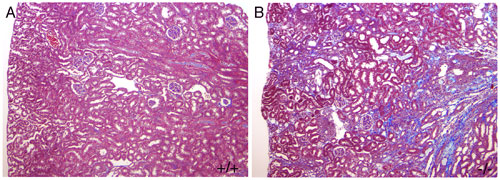
Figure 5 Mice carrying a null mutation in the KCP gene (B) are prone to getting renal fibrosis after injury. This is shown by the blue staining extracellular matrix deposits in B.
 ON THE COVER
ON THE COVER
Breast team reviewing a patient's slide. (From left to right) Ghassan Allo, Fellow; Laura Walters, Clinical Lecturer; Celina Kleer, Professor. See Article 2014Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Autopsy Technician draws blood while working in the Wayne County morgue. See Article 2016Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Sriram Venneti, MD, PhD and Postdoctoral Fellow, Chan Chung, PhD investigate pediatric brain cancer. See Article 2017Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Director of the Neuropathology Fellowship, Dr. Sandra Camelo-Piragua serves on the Patient and Family Advisory Council. 2018Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Residents Ashley Bradt (left) and William Perry work at a multi-headed scope in our new facility. 2019Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Kristine Konopka (right) instructing residents while using a multi-headed microscope. 2020Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Patient specimens poised for COVID-19 PCR testing. 2021Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Pantanowitz demonstrates using machine learning in analyzing slides. 2022Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
(Left to Right) Drs. Angela Wu, Laura Lamps, and Maria Westerhoff. 2023Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Illustration representing the various machines and processing used within our labs. 2024Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|

MLabs, established in 1985, functions as a portal to provide pathologists, hospitals. and other reference laboratories access to the faculty, staff and laboratories of the University of Michigan Health System’s Department of Pathology. MLabs is a recognized leader for advanced molecular diagnostic testing, helpful consultants and exceptional customer service.