

The development of a single cell into a complex, multi-cellular organism requires a precise genetic network to activate specific genes in a spatial and temporal manner. Transcription factors can respond to positional cues in the embryo to control the differentiation and proliferation of stem cells for particular tissues. The Pax family of DNA binding proteins are absolutely required, and in one case sufficient, for the development of a variety of structures such as the eye, kidney, vertebral column , and the nervous system. In developing tissues, cells proliferate rapidly prior to that terminal differentiation. Many types of human diseases result from aberrant proliferation of cells that appear dedifferentiated, assuming a more embryonic phenotype. Consistent with their embryonic photype, such dedifferntiated cells can exhibit enhanced migration or invasion into surrounding tissues.
The Pax2 gene provides a paradigm for understanding how a transcription factor that is required for the development of a tissue can also contribute to a variety of diseases if its activity is not precisely regulated. Pax2 is transiently expressed in the developing renal epithelium, yet has adverse effects if expressed into adulthood. Pax2 expression persists in renal cell carcinoma and Wilms' tumor, as well as infantile and adult poly cystic kidney disease. We have shown a fundamental role of rPax2 in the specification of renal epithelia from intermediate mesodermal stem cells. Pax2 is essential for transducing inductive signals in the developing kidney, yet the biochemical mechanisms underlying Pax2 function remain obscure.
Current projects address the biochemical mechanisms of Pax protein function. Previously, we have shown that the Pax2 transactivation domain is phosphorylated by the c-Jun N-terminal kinase (JNK to enhance transactivation activity. Furthermore, phosphorylation by JNK is blocked by the Pax2 interacting protein Grg4. Grg4 is a member of the Groucho family of transcriptional co-repressor proteins and specifically blocks Pax2 phoshorylation, once Pax2 is bound to its DNA recognition site. We have also identified a novel nuclear protein, PTIP, with homology to tumor suppressor and cell cycle control proteins. We have generated PTIP null mutants and show that PTIP is an essential factor for early development and progression of cells through mitosis.
Pax2 phosphorylation occurs in response to signaling molecules known to have critical roles in renal development. Furthermore, signaling pathways activated after renal injury, when Pax2 expression is detected in regenerating proximal tubules, are also able to phosphorylate the Pax2 activation domain. We hypothesize that Pax2 is positioned at a signaling node where it is able to transduce input from inductive signals during development to activate the renal epithelial specific genetic program. The activity of Pax2 in response to external signaling factors is controlled by modification of the transactivation domain and specific interactions with other cellular factors.
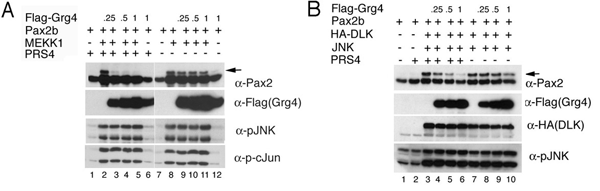
Figure 1 Grg4 inhibits Pax2 phosphorylation in a DNA dependant manner. A) Phosphorylation of Pax2 by the upstream kinase MEKK1 is inhibited with increasing levels of expression of Grg4 when the Pax2 binding site PRS4 is present. B) Phosphorylation of Pax2 by DLK is also inhibited by Grg4 when the PRS4 sequences are present. From Cai et al. submitted.
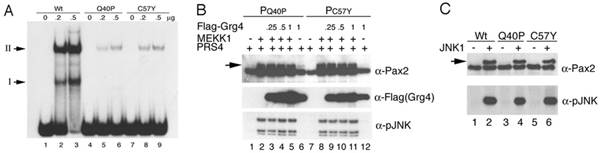
Figure 2 A) Point mutations Q40p and C57Y significantly suppress Pax2 DNA binding activity in an EMSA. B) Tranfection of Q40P and C57Y with MEKK1, PRS4, and increasing amounts of Grg4. Note no effect of Grg4 on levels of P-Pax2 (arrow). C) Both Q40P and C57Y are still good substrates for JNK in an in vitro kinase reaction. From Cai et al. submitted.
We are working on the following projects with regard to Pax Protein function:
1. Defining the serine and/or thronine residues phosphorylated by JNK within the Pax2 activation domain.
2. Characterization of the Phospho-Pax2 specific expression pattern during development and in response to extrinsic signals.
3. Define the interactions among Pax2, Frf4, and PTIP in vitro and in vivo.
4. Generation of a tissue specific PTIP null mutant to study the role of PTIP in kidney.
 ON THE COVER
ON THE COVER
Breast team reviewing a patient's slide. (From left to right) Ghassan Allo, Fellow; Laura Walters, Clinical Lecturer; Celina Kleer, Professor. See Article 2014Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Autopsy Technician draws blood while working in the Wayne County morgue. See Article 2016Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Sriram Venneti, MD, PhD and Postdoctoral Fellow, Chan Chung, PhD investigate pediatric brain cancer. See Article 2017Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Director of the Neuropathology Fellowship, Dr. Sandra Camelo-Piragua serves on the Patient and Family Advisory Council. 2018Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Residents Ashley Bradt (left) and William Perry work at a multi-headed scope in our new facility. 2019Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Kristine Konopka (right) instructing residents while using a multi-headed microscope. 2020Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Patient specimens poised for COVID-19 PCR testing. 2021Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Pantanowitz demonstrates using machine learning in analyzing slides. 2022Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
(Left to Right) Drs. Angela Wu, Laura Lamps, and Maria Westerhoff. 2023Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Illustration representing the various machines and processing used within our labs. 2024Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|

MLabs, established in 1985, functions as a portal to provide pathologists, hospitals. and other reference laboratories access to the faculty, staff and laboratories of the University of Michigan Health System’s Department of Pathology. MLabs is a recognized leader for advanced molecular diagnostic testing, helpful consultants and exceptional customer service.