Study from Venneti Lab on DIPGs Published in Cancer Cell
By Sriram Venneti | August 17 2020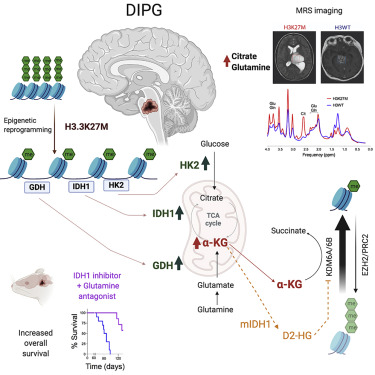 In a new study published in Cancer Cell, Chan Chung et al. from the Venneti laboratory have discovered a “catch 22”-like phenomena, where epigenetic and metabolic pathways feed one another, in a childhood brain tumor called Diffuse Intrinsic Pontine Gliomas (DIPG).
In a new study published in Cancer Cell, Chan Chung et al. from the Venneti laboratory have discovered a “catch 22”-like phenomena, where epigenetic and metabolic pathways feed one another, in a childhood brain tumor called Diffuse Intrinsic Pontine Gliomas (DIPG).
DIPGs are fatal childhood brain tumors that occur mainly in children under 10 years of age. Because they arise within the brainstem region of the brain, surgical resection is not possible and these tumors are invariably devastating and fatal.
DIPGs bear unique mutations in the epigenetic machinery, where histone H3 is mutated. The mutation replaces a lysine (K) residue at position 27 to methionine (M, referred to at H3K27M). Chung et al. discovered that the H3K27M mutations epigenetically modify the genome to increase levels of enzymes related to glycolysis and citric acid cycle metabolism. This increase in metabolism provided fuel for uncontrolled proliferation of tumor cells.
In unexpected results, they found that these metabolic pathways also regulated epigenetic pathways. DIPG cells showed high levels of citric acid cycle metabolite alpha-ketoglutarate. Alpha-ketoglutarate is a critical co-factor for enzymes that demethylate histone and DNA. In a feed-forward “catch-22”-like mechanism, they found that H3K27M mutations produce more alpha-ketoglutarate that, in turn, maintained the preferred epigenetic state of these cells. Isocitrate dehydrogenase (IDH) mutations found in adults show the opposite pattern. They found that H3K27M and IDH mutations are mutually exclusive and were experimentally synthetic lethal.
Using small-molecule strategies, Chung et al. discovered that interrupting this integrated epigenetic and metabolic pathway showed promise in preclinical models of DIPGs. The lab is excited to provide new mechanistic insights into the biology of fatal DIPGs and hopes that these results will aid in the development of a therapeutic that can one day help to provide effective treatments for this incurable childhood brain cancer.
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
