Targeting a metabolite as a therapy for childhood ependymomas
By Sriram Venneti | February 4Ependymomas are the third most common pediatric brain tumor, affecting roughly 250 children in the United States each year. Most cases are diagnosed in children eight years old or younger. Despite decades of research, current treatments extend survival but rarely cure the disease.
A new study published in Nature by Dr. Sriram Venneti’s laboratory at the University of Michigan’s Department of Pathology and the Chad Carr Pediatric Brain Tumor Center has identified a surprising driver of ependymoma growth: a molecule called itaconate. The discovery opens the door to new therapeutic strategies for this aggressive childhood cancer.
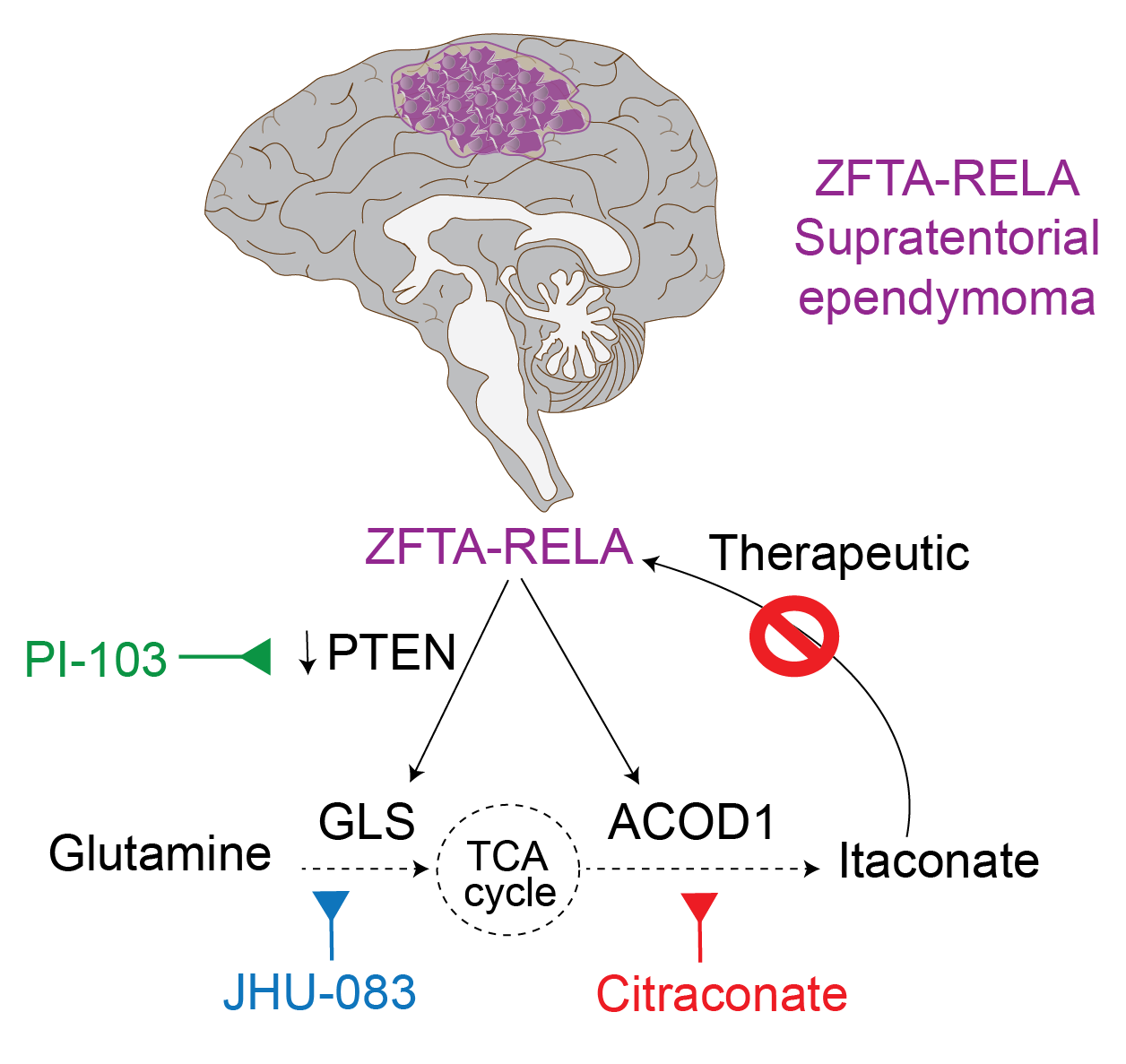 More than 80% of ependymomas arising in the upper brain contain a cancer‑causing protein fusion known as ZFTA‑RELA. These tumors are termed ZFTA-fusion ependymomas. Using ZFTA-fusion animal models and tumor cell lines derived from both patients and mice, the researchers found that ependymomas unexpectedly produce the metabolite itaconate. This is surprising because itaconate is typically generated by macrophage through the enzyme ACOD1 in response to infection. When ACOD1 was blocked in ZFTA-ependymoma mouse models, tumor growth in the brain was dramatically reduced.
More than 80% of ependymomas arising in the upper brain contain a cancer‑causing protein fusion known as ZFTA‑RELA. These tumors are termed ZFTA-fusion ependymomas. Using ZFTA-fusion animal models and tumor cell lines derived from both patients and mice, the researchers found that ependymomas unexpectedly produce the metabolite itaconate. This is surprising because itaconate is typically generated by macrophage through the enzyme ACOD1 in response to infection. When ACOD1 was blocked in ZFTA-ependymoma mouse models, tumor growth in the brain was dramatically reduced.
Further experiments revealed that itaconate forms a positive feedback pathway with the ZFTA‑RELA fusion, with each reinforcing the other to sustain tumor growth. This pathway depends on the amino acid glutamine, which fuels itaconate production. Disrupting this pathway lowered ZFTA‑RELA levels and caused tumors to shrink in mouse models. The team is now collaborating with the Pediatric Neuro‑Oncology Consortium to develop a clinical trial aimed at targeting the itaconate pathway in children with ependymomas.
Additional authors: Siva Kumar Natarajan, Joanna Lum, James Haggerty Skeans, Minal Nenwani, Sanjana Eyunni, Mateus Mota, Jill M. Bayliss, Akash Deogharkar, Erin Taya Hamanishi, Matthew Pun1, Stefan R. Sweha, Simon Hoffman, Eleanor Young, Qiuyang Zhang, Rijul Mehta, Olamide Animasahun, Pranav Narayanan, Sushanth Sunil, Abhijit Parolia, Peter Sajjakulnukit, Pooja Panwalkar, Robert Doherty, Madison Clausen, Derek Dang, Debra Hawes, Fusheng Yang, Mariarita Santi, Alexander R. Judkins, Yelena Wilson, Thomas Vigil, Andrea Franson, Richard M. Mortensen, Tatsuya Ozawa, Andrea Griesinger, Eric C. Holland, Nicholas K. Foreman, Kulandaimanuvel Antony Michaelraj, Sameer Agnihotri, Michael Taylor, Richard J. Gilbertson, Carl Koschmann, Arul M. Chinnaiyan, Costas A. Lyssiotis, and Deepak Nagrath.
Funding/disclosures: This work was supported by the Sontag Foundation; Clinical Scientist Development Award – Doris Duke Charitable Foundation; Hyundai Hope On Wheels Foundation; NINDS R01NS110572; NCI R01CA261926; the Julie Taubman Reys Emerging Scholar Award from the University of Michigan Taubman Institute; and a fellowship from The Robert Connor Dawes Foundation/CERN Foundation/National Brain Tumor Society. Additional support for the Venneti lab came from Mathew Larson, Sidney Kimmel, St. Baldrick’s, Claire McKenna, Chad Tough, Alex’s Lemonade Stand, Storm The Heavens, and the University of Michigan Chad Carr Pediatric Brain Tumor Initiative. Natarajan received support from the Momental Foundation, the Michigan Pioneer Fellows Program, the ChadTough Defeat DIPG, the Alvin L. Glick Foundation, Alex’s Lemonade Stand Foundation Young Investigator Award (#1454502), and the AACR‑SONTAG Foundation Brain Cancer Research Fellowship (#25‑40‑78‑NATA). Koschmann acknowledges NIH Grants R01‑NS119231 and R01‑NS124607, and DOD Grant CA201129P1. Chinnaiyan acknowledges NIH Grant R35CA231996 and is a Howard Hughes Medical Institute Investigator, A. Alfred Taubman Scholar, and American Cancer Society Professor.
Paper cited: “ZFTA‑RELA ependymomas produce itaconate to epigenetically sustain pathogenic fusion expression.”
https://www.nature.com/articles/s41586-025-10005-1
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
