

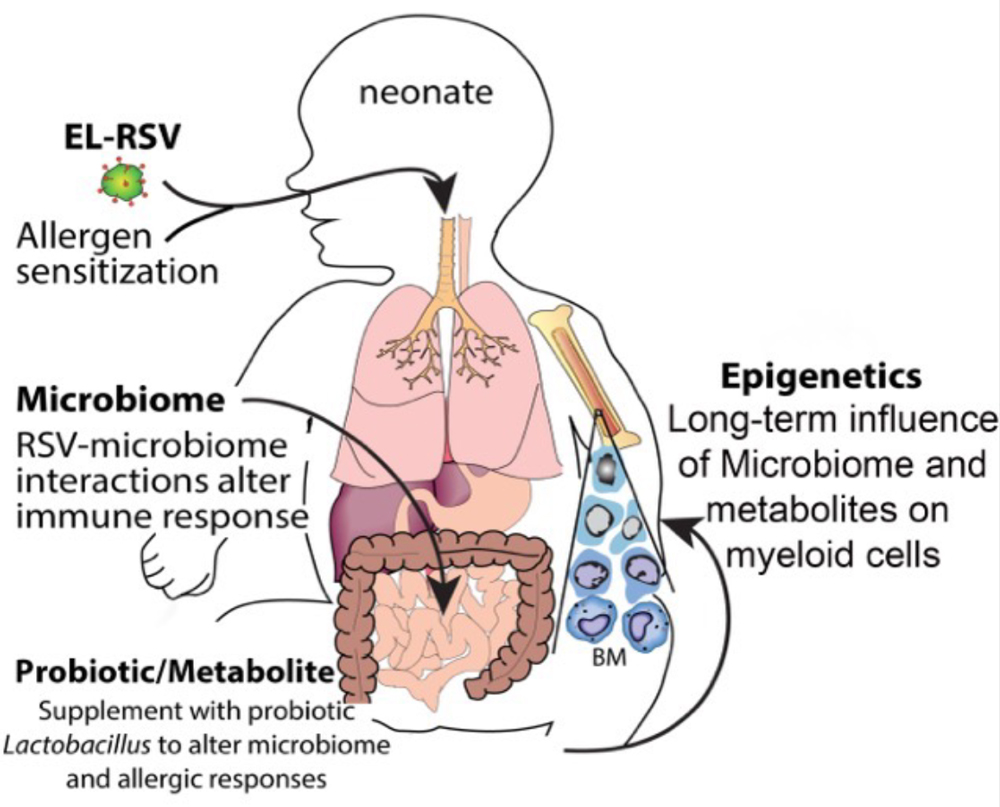
 Over the past several years, our laboratory research has been focused on the immunologic mechanisms involved in the development of asthma and respiratory virus infections. The basic research in the lab has identified numerous targets that are being pursued for alleviating chronic pulmonary disease and at the same time we continue to gain a better understanding of how pulmonary immune responses develop. We have established animal models of pulmonary disease that allow us to ask basic mechanistic questions as well as perform pre-clinical assessments of pharmacologic targets. Our model of allergic airway disease that recapitulates several clinical features of allergic asthma is induced using a cockroach skin test antigen that elicits a strong Th2 skewed response with characteristic eosinophil accumulation and mucus overproduction, as depicted below. The areas investigated using this airway model include mast cell biology, eosinophilic inflammation, dendritic cell biology, and T lymphocyte activation and differentiation. The proper development of the immune system in infants is central to establishing a balanced response that reacts appropriately to infectious stimuli but does not develop pathogenic responses. One of the first signs of an inappropriate and potentially pathogenic immune response in children is manifested by Respiratory Syncytial Virus (RSV) infection, which is the most prominent cause of childhood hospitalization. We have demonstrated that early-life RSV (EL-RSV) infection of the lungs modifies the development of systemic immune responses in neonates that can lead to exacerbated asthmatic responses later in life.
Over the past several years, our laboratory research has been focused on the immunologic mechanisms involved in the development of asthma and respiratory virus infections. The basic research in the lab has identified numerous targets that are being pursued for alleviating chronic pulmonary disease and at the same time we continue to gain a better understanding of how pulmonary immune responses develop. We have established animal models of pulmonary disease that allow us to ask basic mechanistic questions as well as perform pre-clinical assessments of pharmacologic targets. Our model of allergic airway disease that recapitulates several clinical features of allergic asthma is induced using a cockroach skin test antigen that elicits a strong Th2 skewed response with characteristic eosinophil accumulation and mucus overproduction, as depicted below. The areas investigated using this airway model include mast cell biology, eosinophilic inflammation, dendritic cell biology, and T lymphocyte activation and differentiation. The proper development of the immune system in infants is central to establishing a balanced response that reacts appropriately to infectious stimuli but does not develop pathogenic responses. One of the first signs of an inappropriate and potentially pathogenic immune response in children is manifested by Respiratory Syncytial Virus (RSV) infection, which is the most prominent cause of childhood hospitalization. We have demonstrated that early-life RSV (EL-RSV) infection of the lungs modifies the development of systemic immune responses in neonates that can lead to exacerbated asthmatic responses later in life.
In addition, the EL-RSV infection leads to long-term changes in the microbiome that accompany the altered immune responses. These findings reflect clinical data from infants and indicate that both lung and gut microbiomes are altered in infants with severe RSV infections. However, whether and how the microbiome alters the host immune and physiologic response locally in the lungs and/or systemically is unknown and is being actively addressed. We have also established that the early events induced by RSV infection modify the progression of allergic responses later in life through the long-term alteration of immune cell phenotypes by epigenetic mechanisms and accompanying lung structure/function alterations. The investigation of the mechanisms that govern these responses will establish new paradigms and will fill in a significant gap in our understanding of the developing neonatal immune system and pulmonary disease. Our previous studies have helped to link persistent innate immune cells, DCs and ILC2, to the development of a pro-asthmatic, Th2 lung environment later in life. Our published data establish that RSV responses and changed microbiome are associated with altered systemic metabolite profiles and together alter lung function long term. Our data further demonstrates probiotic supplementation with Lactobacillus johnsonii can attenuate the altered long-term lung function changes. Altogether, our studies attempt to highlight how early life environmental factors can shape ongoing and chronic disease by systemic immune modulatory events.
 ON THE COVER
ON THE COVER
Breast team reviewing a patient's slide. (From left to right) Ghassan Allo, Fellow; Laura Walters, Clinical Lecturer; Celina Kleer, Professor. See Article 2014Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Autopsy Technician draws blood while working in the Wayne County morgue. See Article 2016Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Sriram Venneti, MD, PhD and Postdoctoral Fellow, Chan Chung, PhD investigate pediatric brain cancer. See Article 2017Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Director of the Neuropathology Fellowship, Dr. Sandra Camelo-Piragua serves on the Patient and Family Advisory Council. 2018Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Residents Ashley Bradt (left) and William Perry work at a multi-headed scope in our new facility. 2019Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Kristine Konopka (right) instructing residents while using a multi-headed microscope. 2020Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Patient specimens poised for COVID-19 PCR testing. 2021Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Pantanowitz demonstrates using machine learning in analyzing slides. 2022Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
(Left to Right) Drs. Angela Wu, Laura Lamps, and Maria Westerhoff. 2023Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Illustration representing the various machines and processing used within our labs. 2024Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|

MLabs, established in 1985, functions as a portal to provide pathologists, hospitals. and other reference laboratories access to the faculty, staff and laboratories of the University of Michigan Health System’s Department of Pathology. MLabs is a recognized leader for advanced molecular diagnostic testing, helpful consultants and exceptional customer service.