Mast Cell Biology & SCF
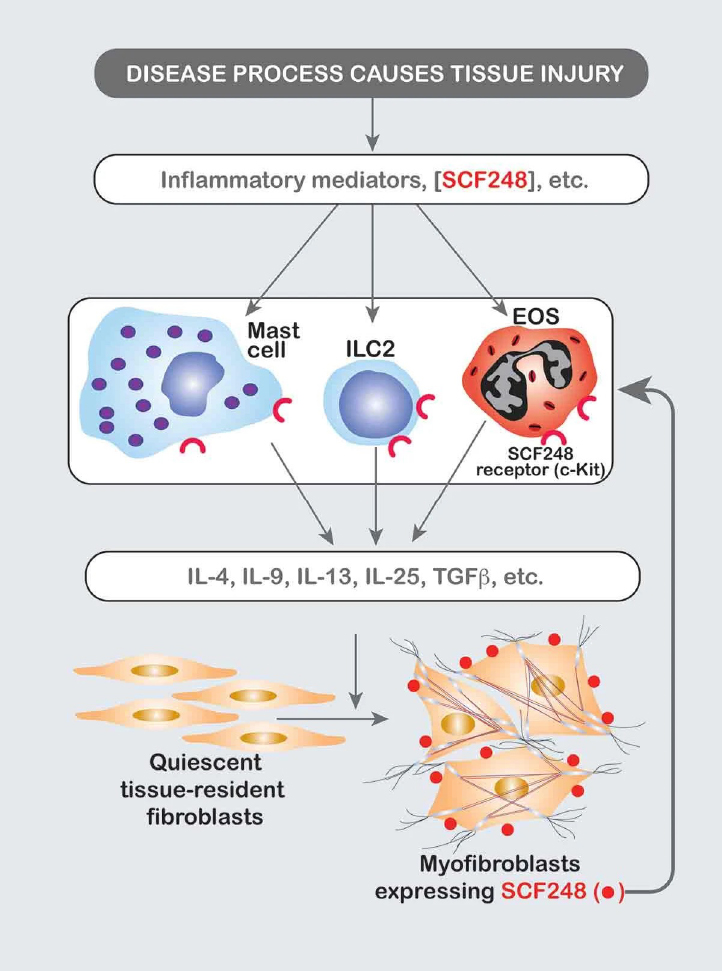 Mast cells are multifunctional cells that can initiate or modulate various inflammatory processes. These cells contain many preformed mediators that, when released, may induce initial and necessary interactions between circulating leukocytes and the endothelium of postcapillary venules. The activation and degranulation of mast cell populations are responses that can be mediated by either antigen-specific, surface bound IgE or by stem cell factor (SCF). IgE-mediated mast cell activation induces immediate mast cell degranulation that constitutes the primary mechanism that drives the allergic responses and type I hypersensitivity. During this activation, mast cells release preformed and newly synthesized pro-inflammatory mediators including histamine, heparin, proteases, prostaglandin D2 , leukotriene C4 , and cytokines. In addition, murine mast cells challenged in an IgE-dependent manner or with other stimuli can produce multiple chemokines, including CCL3 (MIP1a), CCL4 (MIP-1b) and CCL2 (MCP-1) that can initiate and perpetuate an inflammatory cascade. Over the years we have identified the central role of mast cells in allergic inflammation and have further identified SCF as a primary target in peripheral tissue as being upregulated during inflammation and mediating chronic mast cell associated responses. Endogenous SCF occurs primarily in two forms, a 248 amino acid (AA) cleavable form (SCF248) and a 220 AA “noncleavable” form (SCF220) that differ by the presence or absence of exon 6 that encodes for protease cleavage site(s). Both isoforms of SCF are inserted into the plasma membrane, with the extracellular domain (ECD) of SCF248 more efficiently cleaved and shed from the surface of the cell during inflammation. While SCF-ECD is abundantly detected in circulation (~800 pg/mL in humans), circulating SCF-ECD is primarily monomeric that cannot cross-link and activate c-kit. SCF isoforms appear to play divergent roles in development, erythropoiesis, and myelopoiesis, where SCF220 may be required during development and erythropoiesis and SCF248 may be required for normal myelopoiesis and mast cell development and/or differentiation. We have furthermore identified that SCF248 is specifically upregulated in peripheral tissues during chronic inflammatory responses and promote the accumulation of mast cells, eosinophils and ILC2, all innate immune cells that express the SCF receptor, c-kit. The subsequent activation of fibroblasts and other non-immune cells by innate cell cytokines would drive disease severity as well as increase more SCF248 in a Feed-forward activation loop. The inhibition specifically of SCF248 with an antibody that recognizes the SCF248 isoform but not the SCF220 isoform, attenuates the development and severity of ongoing allergic and fibrotic disease models. Thus, we believe that targeting the SCF248 isoform will regulate chronic disease without altering the homeostatic functions of c-kit, such as erythropoiesis. (1-22)
Mast cells are multifunctional cells that can initiate or modulate various inflammatory processes. These cells contain many preformed mediators that, when released, may induce initial and necessary interactions between circulating leukocytes and the endothelium of postcapillary venules. The activation and degranulation of mast cell populations are responses that can be mediated by either antigen-specific, surface bound IgE or by stem cell factor (SCF). IgE-mediated mast cell activation induces immediate mast cell degranulation that constitutes the primary mechanism that drives the allergic responses and type I hypersensitivity. During this activation, mast cells release preformed and newly synthesized pro-inflammatory mediators including histamine, heparin, proteases, prostaglandin D2 , leukotriene C4 , and cytokines. In addition, murine mast cells challenged in an IgE-dependent manner or with other stimuli can produce multiple chemokines, including CCL3 (MIP1a), CCL4 (MIP-1b) and CCL2 (MCP-1) that can initiate and perpetuate an inflammatory cascade. Over the years we have identified the central role of mast cells in allergic inflammation and have further identified SCF as a primary target in peripheral tissue as being upregulated during inflammation and mediating chronic mast cell associated responses. Endogenous SCF occurs primarily in two forms, a 248 amino acid (AA) cleavable form (SCF248) and a 220 AA “noncleavable” form (SCF220) that differ by the presence or absence of exon 6 that encodes for protease cleavage site(s). Both isoforms of SCF are inserted into the plasma membrane, with the extracellular domain (ECD) of SCF248 more efficiently cleaved and shed from the surface of the cell during inflammation. While SCF-ECD is abundantly detected in circulation (~800 pg/mL in humans), circulating SCF-ECD is primarily monomeric that cannot cross-link and activate c-kit. SCF isoforms appear to play divergent roles in development, erythropoiesis, and myelopoiesis, where SCF220 may be required during development and erythropoiesis and SCF248 may be required for normal myelopoiesis and mast cell development and/or differentiation. We have furthermore identified that SCF248 is specifically upregulated in peripheral tissues during chronic inflammatory responses and promote the accumulation of mast cells, eosinophils and ILC2, all innate immune cells that express the SCF receptor, c-kit. The subsequent activation of fibroblasts and other non-immune cells by innate cell cytokines would drive disease severity as well as increase more SCF248 in a Feed-forward activation loop. The inhibition specifically of SCF248 with an antibody that recognizes the SCF248 isoform but not the SCF220 isoform, attenuates the development and severity of ongoing allergic and fibrotic disease models. Thus, we believe that targeting the SCF248 isoform will regulate chronic disease without altering the homeostatic functions of c-kit, such as erythropoiesis. (1-22)
Publications
- Ptaschinski C, Zhu D, Fonseca W, Lukacs NW. Stem cell factor inhibition reduces Th2 inflammation and cellular infiltration in a mouse model of eosinophilic esophagitis. Mucosal immunology 2023; 16: 727-739.
- Ptaschinski C, Rasky AJ, Fonseca W, Lukacs NW. Stem Cell Factor Neutralization Protects From Severe Anaphylaxis in a Murine Model of Food Allergy. Frontiers in immunology 2021; 12: 604192.
- Rasky A, Habiel DM, Morris S, Schaller M, Moore BB, Phan S, Kunkel SL, Phillips M, Hogaboam C, Lukacs NW. Inhibition of the stem cell factor 248 isoform attenuates the development of pulmonary remodeling disease. American journal of physiology Lung cellular and molecular physiology 2020; 318: L200-L211.
- Fonseca W, Rasky AJ, Ptaschinski C, Morris SH, Best SKK, Phillips M, Malinczak CA, Lukacs NW. Group 2 innate lymphoid cells (ILC2) are regulated by stem cell factor during chronic asthmatic disease. Mucosal immunology 2019; 12: 445-456.
- Ding L, Dolgachev V, Wu Z, Liu T, Nakashima T, Wu Z, Ullenbruch M, Lukacs NW, Chen Z, Phan SH. Essential role of stem cell factor-c-Kit signalling pathway in bleomycin-induced pulmonary fibrosis. The Journal of pathology 2013; 230: 205-214.
- Dolgachev VA, Ullenbruch MR, Lukacs NW, Phan SH. Role of stem cell factor and bone marrow-derived fibroblasts in airway remodeling. The American journal of pathology 2009; 174: 390-400.
- Dolgachev V, Petersen BC, Budelsky AL, Berlin AA, Lukacs NW. Pulmonary IL-17E (IL-25) production and IL-17RB+ myeloid cell-derived Th2 cytokine production are dependent upon stem cell factor-induced responses during chronic allergic pulmonary disease. Journal of immunology 2009; 183: 5705-5715.
- Dolgachev V, Berlin AA, Lukacs NW. Eosinophil activation of fibroblasts from chronic allergen-induced disease utilizes stem cell factor for phenotypic changes. The American journal of pathology 2008; 172: 68-76.
- Kumar KG, Barriere H, Carbone CJ, Liu J, Swaminathan G, Xu P, Li Y, Baker DP, Peng J, Lukacs GL, Fuchs SY. Site-specific ubiquitination exposes a linear motif to promote interferon-alpha receptor endocytosis. J Cell Biol 2007; 179: 935-950.
- Dolgachev V, Thomas M, Berlin A, Lukacs NW. Stem cell factor-mediated activation pathways promote murine eosinophil CCL6 production and survival. Journal of leukocyte biology 2007; 81: 1111-1119.
- Berlin AA, Hogaboam CM, Lukacs NW. Inhibition of SCF attenuates peribronchial remodeling in chronic cockroach allergen-induced asthma. Lab Invest 2006; 86: 557-565.
- Berlin AA, Lincoln P, Tomkinson A, Lukacs NW. Inhibition of stem cell factor reduces pulmonary cytokine levels during allergic airway responses. Clin Exp Immunol 2004; 136: 15-20.
- Oliveira SH, Lukacs NW. Stem cell factor: a hemopoietic cytokine with important targets in asthma. Curr Drug Targets Inflamm Allergy 2003; 2: 313-318.
- Oliveira SH, Taub DD, Nagel J, Smith R, Hogaboam CM, Berlin A, Lukacs NW. Stem cell factor induces eosinophil activation and degranulation: mediator release and gene array analysis. Blood 2002; 100: 4291-4297.
- Oliveira SH, Lukacs NW. Stem cell factor and igE-stimulated murine mast cells produce chemokines (CCL2, CCL17, CCL22) and express chemokine receptors. Inflammation research : official journal of the European Histamine Research Society [et al] 2001; 50: 168-174.
- Oliveira SH, Hogaboam CM, Berlin A, Lukacs NW. SCF-induced airway hyperreactivity is dependent on leukotriene production. American journal of physiology Lung cellular and molecular physiology 2001; 280: L1242-1249.
- Klein A, Talvani A, Cara DC, Gomes KL, Lukacs NW, Teixeira MM. Stem cell factor plays a major role in the recruitment of eosinophils in allergic pleurisy in mice via the production of leukotriene B4. Journal of immunology 2000; 164: 4271-4276.
- Bone-Larson CL, Hogaboam CM, Steinhauser ML, Oliveira SH, Lukacs NW, Strieter RM, Kunkel SL. Novel protective effects of stem cell factor in a murine model of acute septic peritonitis. Dependence on MCP-1. The American journal of pathology 2000; 157: 1177-1186.
- Campbell E, Hogaboam C, Lincoln P, Lukacs NW. Stem cell factor-induced airway hyperreactivity in allergic and normal mice. The American journal of pathology 1999; 154: 1259-1265.
- Hogaboam C, Kunkel SL, Strieter RM, Taub DD, Lincoln P, Standiford TJ, Lukacs NW. Novel role of transmembrane SCF for mast cell activation and eotaxin production in mast cell-fibroblast interactions. Journal of immunology 1998; 160: 6166-6171.
- Lukacs NW, Strieter RM, Lincoln PM, Brownell E, Pullen DM, Schock HJ, Chensue SW, Taub DD, Kunkel SL. Stem cell factor (c-kit ligand) influences eosinophil recruitment and histamine levels in allergic airway inflammation. Journal of immunology 1996; 156: 3945-3951.
- Lukacs NW, Kunkel SL, Strieter RM, Evanoff HL, Kunkel RG, Key ML, Taub DD. The role of stem cell factor (c-kit ligand) and inflammatory cytokines in pulmonary mast cell activation. Blood 1996; 87: 2262-2268.


 Mast cells are multifunctional cells that can initiate or modulate various inflammatory processes. These cells contain many preformed mediators that, when released, may induce initial and necessary interactions between circulating leukocytes and the endothelium of postcapillary venules. The activation and degranulation of mast cell populations are responses that can be mediated by either antigen-specific, surface bound IgE or by stem cell factor (SCF). IgE-mediated mast cell activation induces immediate mast cell degranulation that constitutes the primary mechanism that drives the allergic responses and type I hypersensitivity. During this activation, mast cells release preformed and newly synthesized pro-inflammatory mediators including histamine, heparin, proteases, prostaglandin D2 , leukotriene C4 , and cytokines. In addition, murine mast cells challenged in an IgE-dependent manner or with other stimuli can produce multiple chemokines, including CCL3 (MIP1a), CCL4 (MIP-1b) and CCL2 (MCP-1) that can initiate and perpetuate an inflammatory cascade. Over the years we have identified the central role of mast cells in allergic inflammation and have further identified SCF as a primary target in peripheral tissue as being upregulated during inflammation and mediating chronic mast cell associated responses. Endogenous SCF occurs primarily in two forms, a 248 amino acid (AA) cleavable form (SCF248) and a 220 AA “noncleavable” form (SCF220) that differ by the presence or absence of exon 6 that encodes for protease cleavage site(s). Both isoforms of SCF are inserted into the plasma membrane, with the extracellular domain (ECD) of SCF248 more efficiently cleaved and shed from the surface of the cell during inflammation. While SCF-ECD is abundantly detected in circulation (~800 pg/mL in humans), circulating SCF-ECD is primarily monomeric that cannot cross-link and activate c-kit. SCF isoforms appear to play divergent roles in development, erythropoiesis, and myelopoiesis, where SCF220 may be required during development and erythropoiesis and SCF248 may be required for normal myelopoiesis and mast cell development and/or differentiation. We have furthermore identified that SCF248 is specifically upregulated in peripheral tissues during chronic inflammatory responses and promote the accumulation of mast cells, eosinophils and ILC2, all innate immune cells that express the SCF receptor, c-kit. The subsequent activation of fibroblasts and other non-immune cells by innate cell cytokines would drive disease severity as well as increase more SCF248 in a Feed-forward activation loop. The inhibition specifically of SCF248 with an antibody that recognizes the SCF248 isoform but not the SCF220 isoform, attenuates the development and severity of ongoing allergic and fibrotic disease models. Thus, we believe that targeting the SCF248 isoform will regulate chronic disease without altering the homeostatic functions of c-kit, such as erythropoiesis. (1-22)
Mast cells are multifunctional cells that can initiate or modulate various inflammatory processes. These cells contain many preformed mediators that, when released, may induce initial and necessary interactions between circulating leukocytes and the endothelium of postcapillary venules. The activation and degranulation of mast cell populations are responses that can be mediated by either antigen-specific, surface bound IgE or by stem cell factor (SCF). IgE-mediated mast cell activation induces immediate mast cell degranulation that constitutes the primary mechanism that drives the allergic responses and type I hypersensitivity. During this activation, mast cells release preformed and newly synthesized pro-inflammatory mediators including histamine, heparin, proteases, prostaglandin D2 , leukotriene C4 , and cytokines. In addition, murine mast cells challenged in an IgE-dependent manner or with other stimuli can produce multiple chemokines, including CCL3 (MIP1a), CCL4 (MIP-1b) and CCL2 (MCP-1) that can initiate and perpetuate an inflammatory cascade. Over the years we have identified the central role of mast cells in allergic inflammation and have further identified SCF as a primary target in peripheral tissue as being upregulated during inflammation and mediating chronic mast cell associated responses. Endogenous SCF occurs primarily in two forms, a 248 amino acid (AA) cleavable form (SCF248) and a 220 AA “noncleavable” form (SCF220) that differ by the presence or absence of exon 6 that encodes for protease cleavage site(s). Both isoforms of SCF are inserted into the plasma membrane, with the extracellular domain (ECD) of SCF248 more efficiently cleaved and shed from the surface of the cell during inflammation. While SCF-ECD is abundantly detected in circulation (~800 pg/mL in humans), circulating SCF-ECD is primarily monomeric that cannot cross-link and activate c-kit. SCF isoforms appear to play divergent roles in development, erythropoiesis, and myelopoiesis, where SCF220 may be required during development and erythropoiesis and SCF248 may be required for normal myelopoiesis and mast cell development and/or differentiation. We have furthermore identified that SCF248 is specifically upregulated in peripheral tissues during chronic inflammatory responses and promote the accumulation of mast cells, eosinophils and ILC2, all innate immune cells that express the SCF receptor, c-kit. The subsequent activation of fibroblasts and other non-immune cells by innate cell cytokines would drive disease severity as well as increase more SCF248 in a Feed-forward activation loop. The inhibition specifically of SCF248 with an antibody that recognizes the SCF248 isoform but not the SCF220 isoform, attenuates the development and severity of ongoing allergic and fibrotic disease models. Thus, we believe that targeting the SCF248 isoform will regulate chronic disease without altering the homeostatic functions of c-kit, such as erythropoiesis. (1-22)