

Chemokines during immune responses
Mediators produced during inflammatory/immune responses dictate the type of response, as well as its magnitude ("quality and quantity"). The profile of cytokines and chemokines produced are responsible for the cell-to-cell communication that facilitates initial recognition of infection or damage. These signals, in turn, communicate with primary lympoid tissues (the thymus and bone marrow) to mobilize inflammatory cells to the bloodstream. At the tissue site, chemokines and orchestrate leukocyte adhesion to vascular endothelium, extravasation, and localization of leukocytes at the site of inflammation. Recruitment of leukocyte populations into inflamed tissues is initiated by cytokine-induced expression of adhesion molecules on vascular endothelium. Chemokines promote the tight adherence of leukocytes to activated endothelium, as well as direct the extravasation of cells into inflamed tissue. Chemokines further coordinate the response of inflammatory effector cells as they arrive in inflamed tissues. The sequential activation and cellular recruitment cascades mediated by cytokines and chemokines are essential for successful resolution of infection or other tissue damage. However, overproduction or dysregulation of these inflammatory mediators can also lead to destructive, pathological consequences.
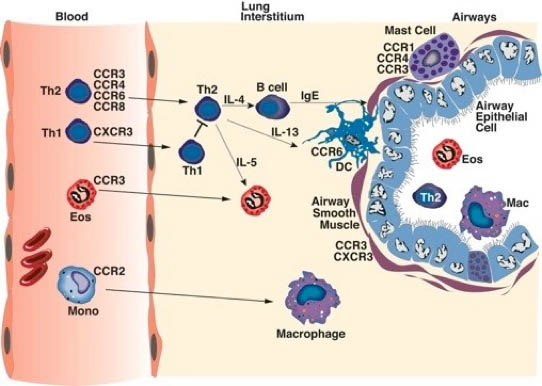
As depicted in the figure above, different cell populations express various and sometimes, specific chemokine receptors that help guide the leukocytes to specific diseased inflammation. In the case as above during allergic airway responses, a Th2 environment produces specific chemokines that are induced by IL-4 and IL-13, such as CCL11 (eotaxin), CCL22 (TARC), CCL20, and CCL1. These ligands bind to specific receptors, CCR3, CCR4, CCR6 and CCR8, respectively, that are found on Th2 cells and eosinophils that are known to be characteristic of an allergic type inflammation. In contrast, cytokines that are associated with viral responses, including type I IFN and IFN gamma induced chemokines, such as CxCL9, CxCL10 and CCL5, which bind to CxCR3 and CCR1. These receptors are found on Th1 cells, Tcytotoxic cells, and NK cells, which are important for virus clearance. Thus, the cytokine/chemokine environment dictates that type of cells that accumulate at the site of inflammation based upon the expression of chemokine receptors found on the leukocyte surface.
Recent Publications on Chemokines and immune responses
1.Kallal LE, Hartigan AJ, Hogaboam CM, Schaller MA, Lukacs NW. Inefficient Lymph node sensitization during Respiratory viral infection promotes IL-17-mediated lung pathology. J Immunol. 2010.
2.Kallal LE, Schaller MA, Lindell DM, Lira SA, Lukacs NW. CCL20/CCR6 blockade enhances immunity to respiratory syncytial virus by impairing recruitment of dendritic cells. Eur. J. Immunol. 183(10):6698-707. 2010.
3.Elhofy A, Depaolo RW, Lira SA, Lukacs NW, and Karpus WJ. 2009. Mice deficient for CCR6 fail to control Chronic experimental autoimmune encephalomyelitis. J. Neuroimmunol.
4.Lindell DM, Lane TE, and Lukacs NW. CXCL10/CXCR3-mediated responses promote immunity to respiratory syncytial virus infection by augmenting dendritic cell and CD8(+) T cell efficacy. Eur. J. Immunol. 2008, 8:2168-79.
5.Schaller MA, Kallal LE, Lukacs NW. A Key Role for CC Chemokine Receptor 1 in T-Cell-Mediated Respiratory Inflammation. Am J Pathol. 2008 172(2):386-94.
6.Dolgachev V, M. Thomas, AA Berlin, and NW Lukacs. Stem cell factor-mediated activation pathways promote murine eosinophil CCL6 production and survival. J. Leuk. Biol. 81(4):1111-9,2007.
7.Smit JJ, Lukacs NW. The missing link: chemokine receptors and tissue matrix breakdown in COPD. Trends Pharmacol Sci. 2006 (11):555-7.
8.Karpus WJ, Kennedy KJ, Fife BT, Bennett JL, Dal Canto MC, Kunkel SL, and Lukacs NW. Anti-CCL2 treatment inhibits Theiler's murine encephalomyelitis virus-induced demyelinating disease. J Neurovirol. 2006 (4):251-61.
9.Allison L. Miller, Craig Gerard, Matthew A. Schaller, Achim D. Gruber, Allison A. Humbles, and Nicholas W. Lukacs. Deletion of CCR1 attenuates pathophysiologic responses during RSV infection. J. Immunol. 2006, 176(4):2562-7.
10.Steven K. Lundy, Sergio A. Lira, Donald N. Cook, Aaron A. Berlin, and Nicholas W. Lukacs. Attenuation of allergen-induced responses in CCR6-/- mice is dependent upon altered pulmonary T lymphocyte activation. J. Immunol. 174(4):2054-60, 2005.
11. Alison E. John, Craig J. Gerard, Allison L. Miller, Aaron A. Berlin, Matthew A. Schaller, Allison A. Humbles, and Nicholas W. Lukacs. Respiratory syncytial virus induced exaggeration of allergic airway disease is dependent upon CCR1-associated immune responses. Eur. J. Immunol. 35(1):108-16, 2004.
12. Alison John, Aaron A. Berlin and Nicholas W. Lukacs. The role of CCL28 in the development of allergic eosinophilic airway inflammation. Am. J. Pathol. 166(2):345-53, 2004.
13. Alison John, Aaron Berlin, and Nicholas W. Lukacs. Effect of RSV-induced CCL5 on allergic airway inflammation. Eur. J. Immunol. Jun;33(6):1677-85, 2003.
14. Allison L. Miller, Robert M. Strieter, Samual Ho, H. Gruber, and N.W. Lukacs. Role of CxCR2 in RSV-induced mucus production and airway hyperreactivity. J. Immunol. 170(6):3348-56, 2003.
15. Lukacs, N.W. Role of chemokines in the pathogenesis of asthma. Nature Reviews Immunology 1:108-116,2001.
16. N.W. Lukacs, D. M. Prosser, S. A. Lira, and D. N. Cook. Requirement for the chemokine receptor CCR6 in allergic pulmonary inflammation. J. Exp. Med. 20;194(4):551-6, 2001.
 ON THE COVER
ON THE COVER
Breast team reviewing a patient's slide. (From left to right) Ghassan Allo, Fellow; Laura Walters, Clinical Lecturer; Celina Kleer, Professor. See Article 2014Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Autopsy Technician draws blood while working in the Wayne County morgue. See Article 2016Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Sriram Venneti, MD, PhD and Postdoctoral Fellow, Chan Chung, PhD investigate pediatric brain cancer. See Article 2017Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Director of the Neuropathology Fellowship, Dr. Sandra Camelo-Piragua serves on the Patient and Family Advisory Council. 2018Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Residents Ashley Bradt (left) and William Perry work at a multi-headed scope in our new facility. 2019Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Kristine Konopka (right) instructing residents while using a multi-headed microscope. 2020Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Patient specimens poised for COVID-19 PCR testing. 2021Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Pantanowitz demonstrates using machine learning in analyzing slides. 2022Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
(Left to Right) Drs. Angela Wu, Laura Lamps, and Maria Westerhoff. 2023Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Illustration representing the various machines and processing used within our labs. 2024Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Rendering of the D. Dan and Betty Khn Health Care Pavilion. Credit: HOK 2025Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|

MLabs, established in 1985, functions as a portal to provide pathologists, hospitals. and other reference laboratories access to the faculty, staff and laboratories of the University of Michigan Health System’s Department of Pathology. MLabs is a recognized leader for advanced molecular diagnostic testing, helpful consultants and exceptional customer service.