

TLRs and Innate Cell Activation
RSV and TLR Activation
Artwork by Robin Kunkel
RSV and TLR Activation
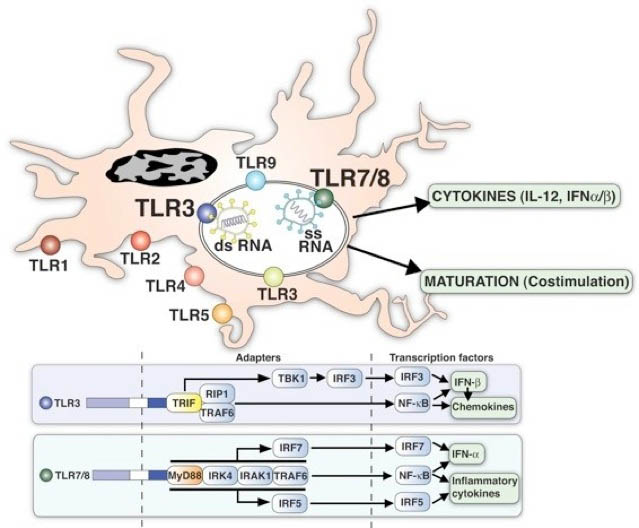
The role of innate immune responses for establishing the most appropriate and least pathologic responses to infectious or deleterious agents is the first line of defense. To deal with this challenge with infectious agents, innate immunity relies on the detection of patterns or conserved molecular motifs unique to various classes of pathogens. The heterogeneity of viral glycoproteins along with their ability to genetically drift from season to season creates even greater challenges for innate immune recognition of viruses. To circumvent these obstacles, the innate immune system has evolved mechanisms to detect characteristics of viral nucleic acids that are either distinct in structure (dsRNA) or subcellular location (ssRNA). Recognition of viral nucleic acids triggers the induction of type I interferons that induce an anti-viral state in virally infected cells and activate immunoregulatory functions in nearby cells. Our lab is most interested in early childhood viral infections and more specifically in respiratory syncytial virus (RSV) that infects most infants by age 2. Since RSV is a ssRNA negative sense virus, both ssRNA and dsRNA species are formed and provide targets for the innate immune system. A subset of pattern recognition receptors includes the toll-like receptors (TLR), which recognize different pathogen-associated molecular patterns (PAMPs) and activate NF-kB and other innate signaling pathways. In particular, TLR3 and TLR7/8 recognize pathogen associated dsRNA and ssRNA species, respectively, and initiate important cytokine mediator pathways for the initiation of the immune responses. These earliest responses are likely critical to establishing the proper immune response.
The RSV F protein has been reported to bind and activate TLR4, the receptor for lipopolysacharide , and distinct polymorphisms to TLR4 has been associated with disease severity in patient populations. Activation of specific chemokines during RSV infection of epithelial cells can be upregulated via TLR3. Our laboratory identified that while CCL5 and CxCL10 can be upregulated via a TLR3 dependent pathway, CxCL8 is dependent upon a MyD88-dependent pathway. Clearly multiple TLR pathways contribute to the generation of an effective anti-RSV response. Interestingly, our findings suggest that RSV infection of TLR3-/- mice (a MyD88-independent mechanism) leads to altered immune environment and promotes increased IL-13 that is associated with increases in mucus production. Thus, alteration of the dsRNA recognition pathway leads to a pathogenic phenotype. Likewise, when MyD88 pathways are deleted in mice an even more pathogenic environment is induced with increased eosinophilia, mucus overexpression and an overall induction of a Th2 cytokine environment. In the present study, we have examined one of the important MyD88-dependent pathways associated with viral RNA detection, TLR7. While the present perception is that pDC are the primary cell population that expresses TLR7, several other cell populations appear to be able to upregulate its expression, including mDC, B cells, and macrophages.
Recent Publications on Innate immune cell responses
1.Lukacs, NW, JJ Smit, S Mukherjee, SB Morris, G Nunez, and DM Lindell. Respiratory virus-induced TLR7 activation controls IL-17 associated increased mucus via IL-23 regulation. 2010. J. Immunol. (In Press).
2.Seki M, Kohno S, Newstead MW, Zeng X, Bhan U, Lukacs NW, Kunkel SL, Standiford TJ. Critical role of IL-1 receptor-associated kinase-M in regulating chemokine-dependent deleterious inflammation in murine influenza pneumonia. J. Immunol. 2010. 184(3):1410-8.
3.Ishii M, Wen H, Corsa CA, Liu T, Coelho AL, Allen RM, Carson WF 4th, Cavassani KA, Li X, Lukacs NW, Hogaboam CM, Dou Y, and Kunkel SL. Epigenetic regulation of macrophage phenotype. 2009 Blood. 114:3244-54.
4.Smit, JJ, Lindell, DM, Boon, L, Kool, M, Lambrecht, BN, and Lukacs, NW. The balance between plasmacytoid DC versus conventional DC determines pulmonary immunity to virus infections. PLoS One, 2008. 3:e1720.
5.Karen A. Cavassani, Makoto Ishii, Haitao Wen, Matthew A. Schaller, Pamela M. Lincoln, Nicholas W. Lukacs, Cory M. Hogaboam, and Steven L. Kunkel. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. 2008. J. Exp. Med. 205:2609-21.
6.Rudd BD, Luker GD, Luker KE, Peebles RS, and Lukacs NW. Type I interferon regulates virus infected dendritic cell maturation and cytokine production. Viral Immunology 20:531-540, 2007.
7.Rudd, BD, MA Schaller, JJ Smit, SL Kunkel, R. Neupane, L. Kelley, AA Berlin, and NW Lukacs. MyD88-Mediated Instructive Signals in Dendritic Cells Regulate Pulmonary Immune Responses during Respiratory Virus Infection. J Immunol. 2007, 178(9):5820-7.
8.Jetse J. Smit, Brian D Rudd, and Nicholas W. Lukacs. Plasmacytoid Dendritic cells modulate the pulmonary immune response and clearance of respiratory syncytial virus. J. Exp. Med. 2006. 203:1153-9.
9.Brian D. Rudd, Jetse J. Smit, Richard A. Flavell, Lena Aleopoulou, Matthew A. Schaller, Achim D. Gruber, Aaron A. Berlin, and Nicholas W. Lukacs. Deletion of TLR3 alters the pulmonary immune environment and mucus production during RSV infection. J. Immunol. 176: 1937-1942, 2006.
10. Brian D. Rudd, Ezra Burstein, Colin Duckett, Xiaoxia Li, and Nicholas W. Lukacs. Differential role of TLR3 in RSV induced chemokine expression. J. Virology 79(6):3350-7, 2005.
 ON THE COVER
ON THE COVER
Breast team reviewing a patient's slide. (From left to right) Ghassan Allo, Fellow; Laura Walters, Clinical Lecturer; Celina Kleer, Professor. See Article 2014Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Autopsy Technician draws blood while working in the Wayne County morgue. See Article 2016Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Sriram Venneti, MD, PhD and Postdoctoral Fellow, Chan Chung, PhD investigate pediatric brain cancer. See Article 2017Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Director of the Neuropathology Fellowship, Dr. Sandra Camelo-Piragua serves on the Patient and Family Advisory Council. 2018Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Residents Ashley Bradt (left) and William Perry work at a multi-headed scope in our new facility. 2019Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Kristine Konopka (right) instructing residents while using a multi-headed microscope. 2020Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Patient specimens poised for COVID-19 PCR testing. 2021Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Pantanowitz demonstrates using machine learning in analyzing slides. 2022Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
(Left to Right) Drs. Angela Wu, Laura Lamps, and Maria Westerhoff. 2023Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Illustration representing the various machines and processing used within our labs. 2024Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|

MLabs, established in 1985, functions as a portal to provide pathologists, hospitals. and other reference laboratories access to the faculty, staff and laboratories of the University of Michigan Health System’s Department of Pathology. MLabs is a recognized leader for advanced molecular diagnostic testing, helpful consultants and exceptional customer service.