

Q: What tubes can I use to draw a Type and Screen?
A: Use a Pink topped EDTA tube. MSC stock number 2869. Mix well to prevent clotting.
Q: If I don't have any pink tubes, can I use another color tube?
A: A large 7 mL Llavender top tube contains the same anticolagulant as the Pink top tube. In order to provide enough specimen for testing, use the larger size Lavender top tube and fill the tube completely.
We cannot accept plastic Red Top tubes as they contain a clot activator. The hospital changed to plastic red top tubes in late November. A plastic tube can be identifed by the light blue diagonal stripes on the tube label and the mold marks on the bottom of the tube. A glass tube is smooth.
Q: Why is blood bank changing to Pink Tubes?
A: The effective date of the change was September 8, 2003. The Blood Bank changed from RED to PINK top tubes. The new tubes are made of plastic so they are safer for patients and staff. . Either one can be drawn before or after the other as they both contain EDTA and they both require mixing immediately after collection. . Blood Cultures are drawn first, then the order from left to right is:
Blood culture bottles; blue; yellow, Red glass, red plastic, green; lavender and pink; and finally gray. For a more extensive discussion of tube collection sequences, see question #6.
Reference: NCCLS Document on Blood Collection.
Q: What tube should I use for Pediatrics?
A: A pink top tube filled with at least 2.0 mL of blood for neonatal patients. Failure to provide enough specimen will delay patient care as a new specimen will need to be collected. In addition, the waste specimen would contribute to the blood loss for the patient.
Reference: Front Cover - Blood Transfusion Policies
Q: What is the minimum amount of blood for adults and pediatric specimens?
A: Adult: 7 mL
Child: (Year Range)
5-12 yr 5 mL
1-5 yr 3 mL
4 mo to 1 yr 2.0 mL
0-4 mo 2.0 mL
Reference: Front Cover - Blood Transfusion Policies
Q: In what order should I draw specimens?
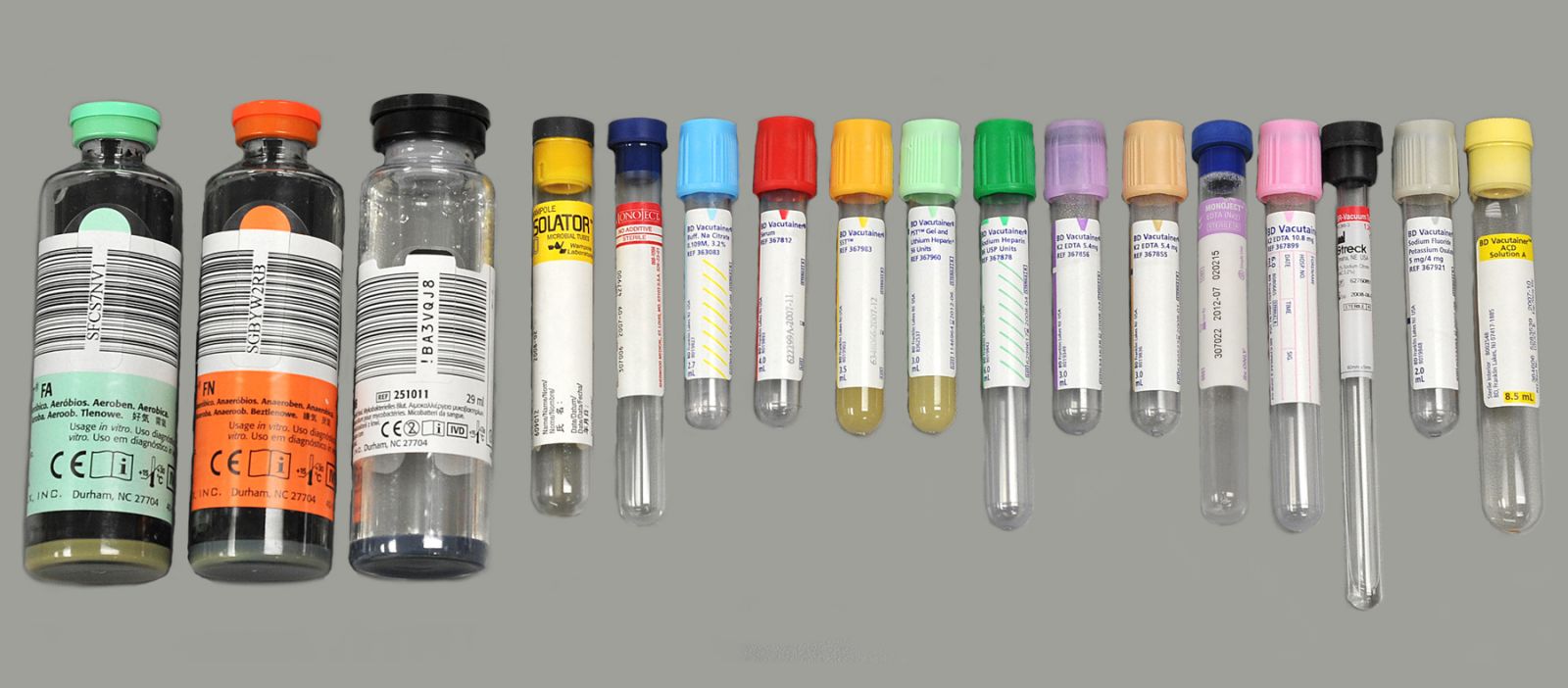
A: Tubes are drawn in the order shown below. This order is only for tubes used at the University of Michigan Hospitals as the stopper color and design changes with the manufacturer. The order within a group is not important. For example, in group 5, either the gold or the red can be drawn first. The navy blue tubes in the photos below are different. The navy tube in group 3 has no additive and the navy tube in group 7 contains Sodium EDTA. The order of draw is set in order to prevent contamination of the blood specimen with anticoagulants that may cause incorrect test results.
#1 Microbiology Blood Culture ,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,#2 Fungus & AFB Culture ....................#3 Navy Blue Top with red label
#4 Coagulation - light blue.......................................$5 Red & Gold - Within this group, it does not matter the order...
# 6 Green Top - Heparin .........................................#7 Navy Blue with lavender label, & pink, lavender, black and tan
Any order within this group.
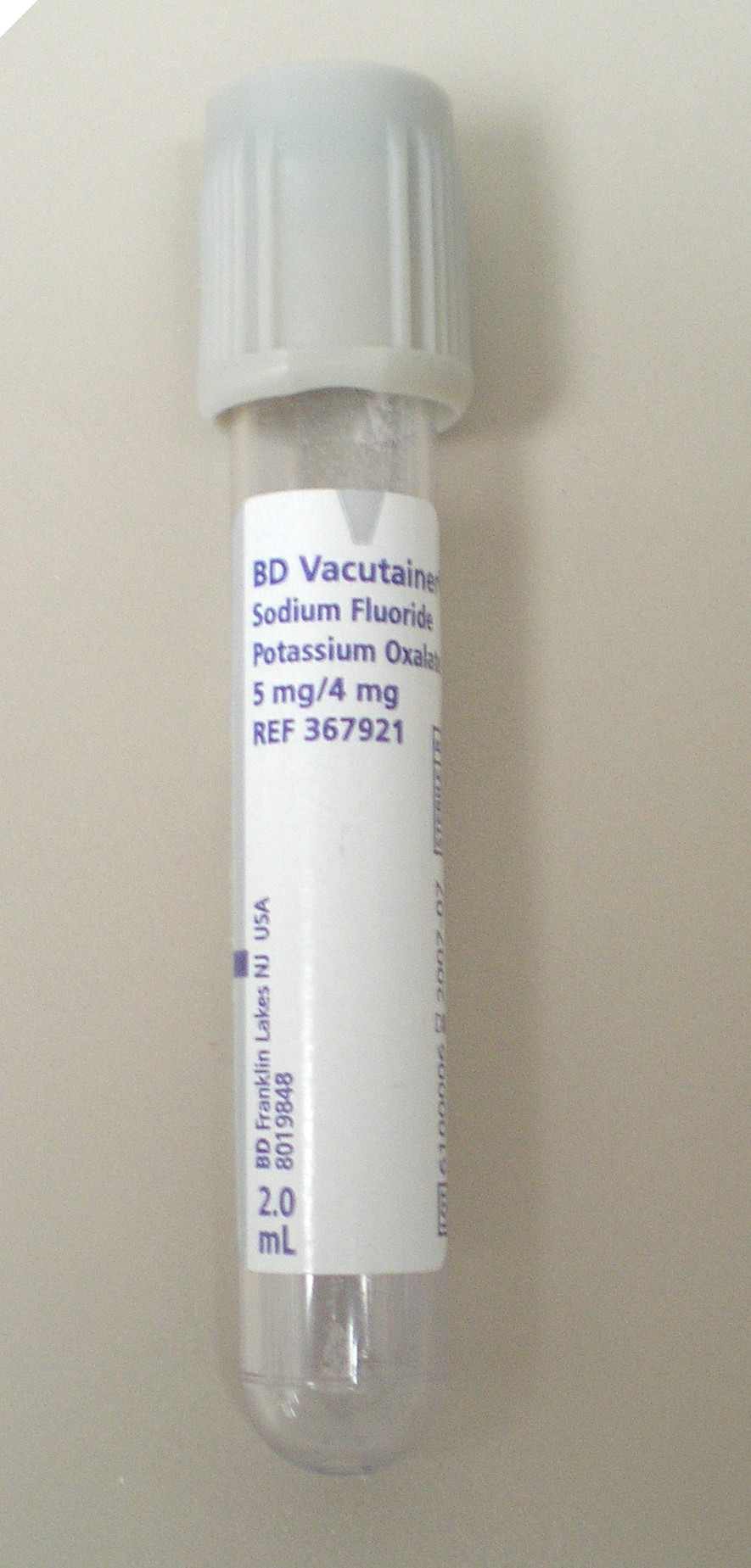
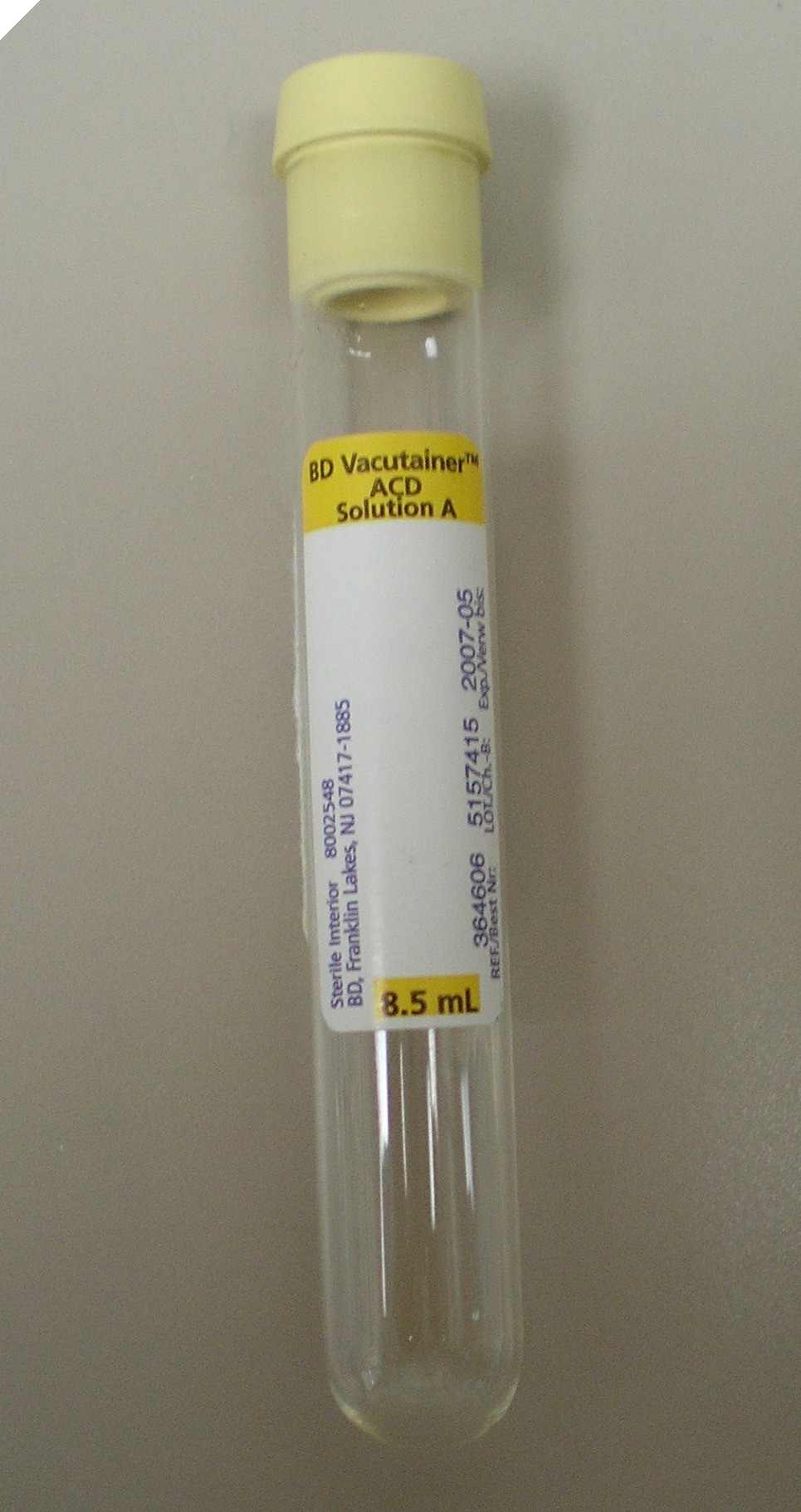
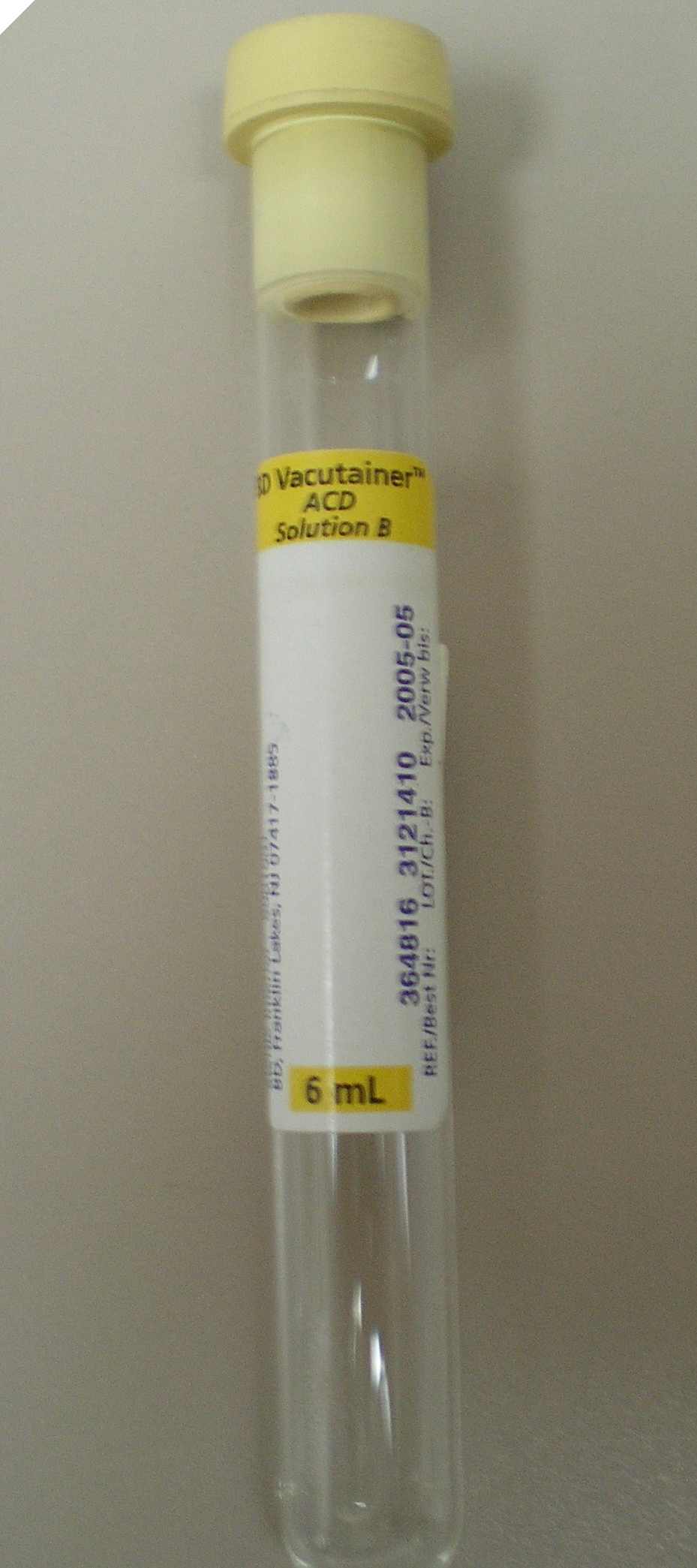
#8 Sodium Floride .....................................................#9 ACD-A ........................................#10 ACD-B
Q: How do I label a tube?
A: Do not flag the label around the middle of the tube.
Use a tube label appropriate for the size of the tube.
Reference: for additional instructions on identifying a patient and labeling a tube for blood bank testing refer to page 4 of the Blood Transfusion Policies Booklet.
Q: How long is a type and screen good?
A: A type and screen specimen is good for 3 days. It is good until 12midnight on the third day. Note that the date of collection is day 0. If a a specimen is collected on Monday red blood cells crossmatched using that specimen may be transfused until midnight on Thursday.
Page 2 Blood Transfusion Policies and Standard Practices
Q: Can I draw a blood specimen while the patient is being transfused?
A: If a patient is receiving a blood transfusion, blood specimens may be collected during the infusion from the other arm where blood is not being infused. If a platelet count or hemoglobin value is being measured, the standard time for a specimen to be collected is 1 hour post infusion. In some cases, a 10 minute posttransfusion pletelet count may be needed to evaluate refractoriness to platelet transfusions. If it is necessary to draw a specimen from the same IV line that fluids are being infused: 1)stop the infusion, 2) collect a tube of blood and discard it as it will be contaminated with IV fluid and test results will not be valid and 3) collect and label tubes as required for testing, and 4) reinitiate the infusion.
Page 288 Phlebotomy Handbook, 4th ed Garza and Becan-McBride, Appleton & Lange, Stamford, CT, 1996.
Q: Why must I throw away the first tube when collecting a blood specimen for laboratory testing from a catheter or central line?
A: The sample is diluted with IV fluid and/or heparin and the results will not be accurate.
Page 288 Phlebotomy Handbook, 4th ed Garza and Becan-McBride, Appleton & Lange, Stamford, CT, 1996
Q: Can Alaris infusion pumps be used with blood components?
A: Yes, the pumps are approved for use with plasma, platelets and red blood cell infusions. There are blood sets for the pump.
Alaris Product Information
Q: How many products can be infused through a line/tubing setup?
A: There is no exact number of units that can transfused through a single blood administration set. Regular blood filters can be used for multiple units. However, transfusion of more than three units of red blood cells through a single tubing set increases the risk of clogging the filter with cellular debris or clots. The filter should be observed at regular intervals during the transfusion for evidence of accumulated debris and changed as needed. The specific manufacturer's instructions for the transfusion set should be followed. Alaris blood tubing should be changed at least every 24 hours accourding to the manufacturer's instructions. Although they are not often used now, Leukocyte-removal filters are intended for use with a single blood component.
Pages 55-56 Blood Transfusion Policies and Standard Practices
Q: What blood products need filters?
A: All blood components must be transfused through a filter. A standard (generally 170-260 micron) filter may be used in most circumstances. The only exception is when blood and blood components arrive in a syringe and are labeled "prefiltered". The blood bank technologist has filtered these components at the time they are drawn into the syringe and the component must be transfused within 4 hours of preparation.
Page 56 Blood Transfusion Policies and Standard Practices
Page 3 Circular of Information for the Use of Blood and Blood Components (2009)
Q: How long can Red Blood Cells and Plasma be outside of a blood bank refrigerator?
A: Updated 11/8/12 If a unit of red blood cells or plasma leaves the blood bank, it should be infused as soon as possible and within 4 hours of leaving the blood bank.
If a unit of blood is returned to the blood bank, the red blood cells or plasma can be reissued under limited circumstances. The red cells and plasma must have been maintained at 1-10C. If a unit is out more than 30 minutes, the temperature has likely exceeded 10C. It is still usable for the original patient for up to 4 hours but cannot be returned for reissue later.
Page 59 Blood Transfusion Policies and Standard Practices
Page 59 Blood Transfusion Policies and Standard Practices
Q: How long can a blood component hang?
A: Once the component is spiked for transfusion it should be infused in no more than 4 hours.
Page 59 Blood Transfusion Policies and Standard Practices
Page 3 #13 Circular of Information for the Use of Blood and Blood Components
Q: How frequently should vital signs be taken during a transfusion?
A: Baseline
Q 15 minutes time
Hourly
One hour post transfusion for inpatients and 30 minutes post transfusion for outpatients
A pretransfusion BP, T, P, R (VS) should be recorded as a baseline for comparison of vital signs obtained during and after a transfusion. Complete VS should be taken 15 minutes after initiating the transfusion and the nurse must stay at the bedside for this first 15 minutes. VS should be repeated 30 minutes after initiation and hourly as long as the component is infusing. Note the time of completion of the transfusion and a repeat set of VS should be obtained after completion.
Page 60 Blood Transfusion Policies and Standard Practices
Page 3 Circular of Information for the Use of Blood and Blood Components (2009)
Q: Can we give blood components with an automated pump (example Alaris, IVAC or ABBOTT pump)?
A: Yes, red blood cells, platelets, and plasma may be administered through an automated pump using specially made pump Blood administration tubing. See manufacturer's recommendations for details.
Pages 56-57 Blood Transfusion Policies and Standard Practices
Q: How fast can we give blood components? Should Platelets be Transfused Slowly?
A: Red Blood Cells over 1-2 hours
Plasma, platelets, and cryo at 10ml per minute or over 20-30 minutes
In a non-urgent situation infuse the first 25ml of blood (proportionately smaller volume for pediatric patients) slowly at no more than 25 drops/minute to allow for recognition of an acute adverse reaction. Complete the transfusion within two hours unless the patient can tolerate only gradual expansion of the intravascular volume. The infusion time should not exceed 4 hours.
Platelets, plasma and cryoprecipitate: The transfusion may be administered as rapidly as the patient can tolerate, usually 30 minutes. In order for a patient to receive maximum benefit platelets need to be transfused rapidly to get control of bleeding. Transfusion as soon as possible after they are available is optimum since platelet function deteriorates during storage.
To assess the effectiveness of the platelet transfusion, a one hour post transfusion platelet count is needed. If the transfusion of platelets occurs over a 3-4 hour time span and the one hour post platelet count is obtained, an accurate assessment of effectiveness of the platelet transfusion is impossible and may lead to recommendation of an inappropriate product.
Page 62 Blood Transfusion Policies and Standard Practices
Page 3 #10 Circular of Information for the Use of Blood and Blood Components
Q: What is the smallest needle we can use to transfuse blood components?
A: The size of the needle is not an issue. The amount of pressure exerted on the red blood cells is the limiting factor. Blood can be infused through the smallest of needles as long as great pressure is not needed to get the blood to flow. Excessive pressure causes lysis of the red blood cells.
Q: If a patient has a fever, can I transfuse blood components?
A: With physician approval, a transfusion may be administered to a febrile patient. However, if a patient is febrile, consideration should be given to postponement of the blood transfusion, since the fever may mask the development of a febrile reaction to the blood component itself.
Page 62 Blood Transfusion Policies and Standard Practices
Q: What is the policy for transporting patients with blood hanging?
A: Patients should not be transported to appointments with blood components infusing unless there is a nurse that can travel with the patient and monitor the patient and the blood. Many clinic and appointment areas are not staffed to monitor a patient while they are receiving blood components.
Q: Can we give Rh positive components to Rh negative patients?
A: RED BLOOD CELLS: Yes, if it is an emergency or if large amounts of blood may be used.
PLATELETS: ABO and Rh compatible platelets will be released for transfusion. If this is not feasible because of limited inventory and /or emergent need for the component, ABO and /or Rh incompatible platelets may be issued. It may be advisable to administer Rho (D) immune globulin to selected Rh-negative patients who receive platelets from Rh-positive donors, since sensitization to red cell antigens may occur from the few red cells present in platelets.
Page Back cover Blood Transfusion Policies and Standard Practices
Page 10 Circular of Information for the Use of Blood and Blood Components
Q: How does a split product read on the form?
A: There are a large number of product abbreviations for blood components. Split units may be described as Part A, Part B, Part 1, Part2, Part 3 or as Divided or Part with the part number following the unit number.
Examples:
ALR1I Apheresis Leukocyte-reduced Red Cell Irradiated Part 1
LRRP Leukocyte-reduced Red Blood Cells, Part
PLTAX Single Donor Platelet, Crossmatched, Part A
FFPL3 Fresh Frozen Plasma Apheresis Part 3
Pages 95-98, Appendix C Blood Transfusion Policies and Standard Practices
Q: If a blood product has been double checked at the bedside and it is removed from the bedside, do I need to double check the blood again when the blood is brought back to the patient's bedside?
A: Yes, a blood product must always be re-checked following the normal procedure if , for any reason, the product is removed from the bedside. If there are signatures already on the form documenting the double check, sign above the original signatures.
Page 60, Blood Transfusion Policies and Standard Practices. July, 2004 version. Immediately Prior to Blood Transfusion.
Q: Can I transfuse different products through regular blood tubing?
A: Yes, unless otherwise stated in the instructions for use on the blood tubing packaging you can transfuse red blood cells, platelets, plasma or cryo through the same filter set. However, the products should be transfused sequentially not simultaneously.
Q: How often should I change blood filters?
A: Blood filter sets should be changed when debris builds up, when the transfusion episode is complete, or every 24 hours, whichever comes first.
Infection Control & Epidemiology Policy "Summary of recommedned frequency of activities related to vascular access devices". 6/30/02
Q: Can I mix different products in the same set at the same time?
A: Unless it is an urgent situation, only one unit should be transfused at one time. Different products should not be transfused at the same time using the same filter set even in cases of emergency. Sepatate filter sets should be used.
Q: Can a PALL white blood cell removal filter be used with an infusion pump?
A: Yes.
PALL product circular and PALL Technical Assistance.
Q: If a product outdates while hanging, is it still good? For how long?
A: The transfusion should be started prior to unit outdate and completed within 4 hours. If it is anticipated that blood or blood components cannot be infused within 4 hours, request that the blood bank divide the unit. The second part of the unit will be stored appropriately in the blood bank until needed.
The 2009 revision of the Circular of Information for the Use of Human Blood and Blood Components, August 2009, on page 3, #13
Revised 2/15/10
Q: What components need to be double checked before transfusion?
A: All Components.
Page 61 Blood Transfusion Policies and Standard Practices
Q: When do you use a Pall leukocyte removal filter?
A: When removal of white blood cells is required and the leukocyte reduction has not been performed prior to use of the component. Patients who require leukocyte-reduced components include:
Immune compromised patients
Patients with repeated febrile transfusion reactions
Patients at risk of acquiring CMV infection
Patients at risk of becoming refractory to platelet transfusions
Bone marrow and stem cell transplant patients
Kidney transplant patients
Page 22 Blood Transfusion Policies and Standard Practices
Q: If I use a Pall leukocyte-removal filter do I need to use a blood administration tubing set as well?
A: A blood administration tubing set is not required if a Pall filter is used since the Pall filter also prevents the infusion of clots.
Q: Is there any written information on how staff could determine how long to run a unit of Red Blood cells for kids? I see adult tranfusoin flow rates in the blood information.
A: All transfusions should be completed within 4 hours and prior to component expiration. If it is anticipated that blood or blood components cannot be infused within 4 hours, they should be divided and stored appropriately in the blood bank until needed. This applies tothe transfusion of both adults and children. The concern is possible bacterial growth in the blood component. Thus, the age of the patient is not the concern.
Page 7# 12 Circular of Information for the Use of Human Blood and Blood Components, August 2000.
Q: How do I handle an emergency transfusion?
Q: How is albumin transfused?
A: A general consent for treatment covers infusion of albumin. Manufacturer's instructions vary as to the requirement for a filter. Our current policy is that no filter is needed. In general, Maximum rates of I.V. infusion after initial volume replacement: 5%: 2-4 mL/minute 25%: 1 mL/minute.
Refer to the manufacturer's instructions for infusion instructions. Updated 5/2/12
Q: Can a double lumen catheter be used to transfuse blood components?
A: Yes. Double and triple lumen catheters are placed in fast flowing vessels and the blood component does not mix with the other fluid in the IV tubing. They may be used for blood infusion unless it is not recommened by the manufacturer.
Q: What are my responsibilities when caring for a patient with a suspected transfusion reaction?
A: Stop the transfusion
Keep IV open at TKO
Double check ID of patient and blood
Notify patient's physician
Notify blood bank
Treat the reaction
Complete the transfusion reaction report form
Page 67-78 Blood Transfusion Policies and Standard Practices
Q: What is a febrile transfusion reaction?
A: Temperature rise of 1.8 F OR 1.0C above baseline
A temperature rise of 1.8 F or 1.0 C from the baseline is considered a febrile reaction that must be worked up. The fever may or may not be accompanied by chills or rigors (shaking chills). The fever may occur during the transfusion or in the immediate posttransfusion period. It must be determined if the fever is related to underlying disease or infection or from an acute hemolytic transfusion reaction. Fever is the most frequent symptom of an acute hemolytic transfusion reaction. The transfusion must be stopped and the physician and the blood bank must be notified. Tylenol may be administered with a physician's order. Severe shaking chills ( rigors) may be controlled by the sedative effect of Benadryl or Demerol. Blood specimens from the patient that may be needed to rule out the acute hemolytic transfusion reaction include two 7 mL Pink top tubes. (Smaller volumes for pediatric patients as requested by the blood bank)
Page 70 Blood Transfusion Policies and Standard Practices
Page 4 Circular of Information for the Use of Blood and Blood Components
Q: What is an urticarial reaction?
A: Hives and itching
Treatment: Benadryl
Foreign plasma proteins in the blood component cause this reaction. A mild urticarial reaction, if not associated with any other symptoms, is generally innocuous. Once the hives have resolved, the transfusion may be resumed with physician approval. Antihistamines may be administered before the blood transfusion as premedication to prevent urticaria for patients with known allergic reactions to blood.
Page 71-72 Blood Transfusion Policies and Standard Practices
Page 4 Circular of Information for the Use of Blood and Blood Components
Q: What is an acute hemolytic reaction?
A: Transfusion of ABO incompatible blood caused by human error.
Signs and symptoms may include: feeling of impending doom, chills, fever, feeling of heat along the vein, lumbar pain, chest pain, tachycardia, hypotension, hemoglobinuria, uncontrollable bleeding
Treatment includes treatment of shock, renal failure, and DIC with IV fluids, vasopressors, and diuretics
The most dreaded complication of blood transfusion is the acute hemolytic reaction in which transfused red cells react with circulating antibody in the recipient with resultant intravascular hemolysis. Transfusion policy dictates that the nurse must stay in the room of a patient receiving blood for the first 15 minutes of the transfusion. Give the blood very slowly infusing no more than approximately 25ml (proportionately smaller volumes for pediatric patients) in this first 15 minutes. This reaction is dose and rate related. The more of the incompatible blood that the patient gets, and the faster they receive it, the worse will be the outcome. This reaction is potentially life threatening, yet completely avoidable as long as proper identification of the patient and the units of blood are performed.
Page 70-71, Blood Transfusion Policies and Standard Practices
Pages 9-10 Circular of Information for the Use of Blood and Blood Components
Q: What is a Delayed Hemolytic Transfusion reaction?
A: CAUSE: Pre-existing low titer antibody in the patient that is undetectable by routine testing. Subsequent transfusion of blood with an antigen specific for this low titer antibody results in hemolysis.
SIGNS AND SYMPTOMS: jaundice, hemoglobinuria, and anemia
TIMEFRAME: 4-8 days or as much as 2 weeks after transfusion
NURSE'S ROLE: give patient post transfusion instructions
TREATMENT: repeat CBC, blood sample for blood bank, transfusion, renal function monitoring, and fluid loading to promote diuresis
A Delayed Hemolytic Reaction is a hemolytic transfusion reaction that occurs about 4-8 days after the blood transfusion. They occur in patients who have developed antibodies from previous transfusion or pregnancy but, at the time of pretransfusion testing, the antibody in question is too weak to be detected by standard procedures. Subsequent transfusion with red cells having the corresponding antigen results in an antibody response and slow extravascular hemolysis of the transfused red cells.
The patient should be informed that this is not a medical emergency but their physician should evaluate the situation.
Page 67 Blood Transfusion Policies and Standard Practices, July 2004 version
Page 5 Circular of Information for the Use of Blood and Blood Components
Q: Who should obtain consent for blood transfusion?
A: The physician and other health care providers who currrently obtain consent for procedures can also obtain consent for transfusion. This does mean that the person obtaining consent has an understanding of the risks and benefits of transfusion. Questions about the process for obtaining consent for blood transfusion and the risks of transfusion should be addressed to one of the Blood Bank Medical Directors. Contact the Blood Bank at 6-6888.
Q: Can a nurse or other health care professional sign as the witness on the Informed Consent form?
A: Yes, signing as witness is only verifying the signature on the form.
Q: Where can I get a copy of the Consent to Receive Blood Transfusion or the Refusal for treatment forms?
A: The Consent to Receive Blood Transfusion is on the back of the Request and Consent to Medical, Surgical and Radiological or Other Procedures form. It can be obtained from Moore Document Solutions form number 2040700. The refusal for treatment form can be found at the following address https://141.214.6.15/bloodbank/releaseform.html.
Q: Is there a time limit on the Consent for Transfusion?
A: A single consent is good for all transfusions during a hospitalization or a course of treatment. A course of treatment begins when the diagnosis is made that necessitates the transfusion and ends when the clinical problem is resolved. This means that one consent will cover all transfusions during one hospital stay. It will also cover transfusions given as an outpatient or during multiple hospital stays if they are part of a treatment course. For instance, for a patient with leukemia one consent can cover many transfusions over the course of years. There is no specific time limit on a consent. We tried to make it as flexible as possible. However, it is advisable to obtain a new consent if there is a significant change in the patient's status, such as: transfer of care to another service a new hospitalization or a new diagnosis.
Reference: On-line copy of Blood Transfusion Policies Chapter 6 and Informed Consent Presentation on Blood Bank web page https://www.pathology.med.umich.edu/bloodbank/default.htm
Q: Should the patient carry a copy of the Consent form if the patient is being tranfused on an outpatient basis?
A: There is a documentation problem with outpatients since the chart may not be available. The Cancer Center maintains a copy of the consent in their shadow chart. Fortunately, outpatients are transfused in only a few areas so it is not an issue for most clinics.
Q: When should a post transfusion sample be collected to monitor the transfusion of Red Blood Cells and Platelets?
A: A 1 hour post transfusion sample can be used for both components.
Q: What should I do if a patient shows me a card from the Blood Bank or Transfusion Service of an outside institution saying the patient has antibodies or problems with blood transfusions?
A: Call the blood bank and advise them of the information on the card. To prevent clerical and communication errors, fax a copy of the card to the Blood Bank 6-6855. Return the card to the patient.
In many cases special blood components will be required for the patient. Failure to communicate preexisting antibodies could result in an immediate or delayed transfusion reaction. Antibodies detected some years ago may not be present at high enough levels to be detected by antibody screening tests and we rely on previous history to prevent an incompatible transfusion.
Blood Transfusion Policies, Chapter 1.
Q: Where can I find more information about RhoGAM®?
A: You can view information about the product and its usage in a power point presentation here.
Q: The unit of Red Blood Cells is labeled as outdating today. What time today does it outdate?
A: The Circular of Information for the Use of Blood Bank Blood Components (page 2 item # 8) indicates that "When the expiration time is not indicated, the product expires at midnight (23:59:59).
Q: Are there ay blood substitutes that can be used for patient who refuse blood transfusion?
A: At present there are no blood substitutes available. There are none in clinical trials that could be obtained for compasstionate use.
Q: Do you perform paternity testing?
A: The University of Michigan Hospitals does not perform paternity testing. Contact the Michigan Department of Human Service for more information, 1-866-540-0008.
Q: Do you have a cord blood bank for future infusions?
A: The University of Michigan does not store cord blood for future infusion. A web search of cord blood banks will provide information on potential storage facilities.
Other Cord Blood Bank Information
 ON THE COVER
ON THE COVER
Breast team reviewing a patient's slide. (From left to right) Ghassan Allo, Fellow; Laura Walters, Clinical Lecturer; Celina Kleer, Professor. See Article 2014Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Autopsy Technician draws blood while working in the Wayne County morgue. See Article 2016Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Sriram Venneti, MD, PhD and Postdoctoral Fellow, Chan Chung, PhD investigate pediatric brain cancer. See Article 2017Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Director of the Neuropathology Fellowship, Dr. Sandra Camelo-Piragua serves on the Patient and Family Advisory Council. 2018Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Residents Ashley Bradt (left) and William Perry work at a multi-headed scope in our new facility. 2019Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Kristine Konopka (right) instructing residents while using a multi-headed microscope. 2020Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Patient specimens poised for COVID-19 PCR testing. 2021Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Dr. Pantanowitz demonstrates using machine learning in analyzing slides. 2022Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
(Left to Right) Drs. Angela Wu, Laura Lamps, and Maria Westerhoff. 2023Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Illustration representing the various machines and processing used within our labs. 2024Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|
 ON THE COVER
ON THE COVER
Rendering of the D. Dan and Betty Khn Health Care Pavilion. Credit: HOK 2025Department Chair |

newsletter
INSIDE PATHOLOGYAbout Our NewsletterInside Pathology is an newsletter published by the Chairman's Office to bring news and updates from inside the department's research and to become familiar with those leading it. It is our hope that those who read it will enjoy hearing about those new and familiar, and perhaps help in furthering our research. CONTENTS
|

MLabs, established in 1985, functions as a portal to provide pathologists, hospitals. and other reference laboratories access to the faculty, staff and laboratories of the University of Michigan Health System’s Department of Pathology. MLabs is a recognized leader for advanced molecular diagnostic testing, helpful consultants and exceptional customer service.