Kim lab identifies the niche and mobilization mechanism for bone marrow innate lymphoid cell progenitors
By Lynn McCain | January 25 2023The laboratory of Dr. Chang Kim, recently published a high-impact study that elucidates the bone marrow niche and mechanisms by which innate lymphoid cells differentiate between those which remain in the bone marrow and those which emigrate to the rest of the body. While T, B, and NK cells are well-known lymphocytes that protect the body from infection and cancer, there are additional lymphocytes that are important for maintaining tissue integrity and the immune system. These are innate lymphoid cells (ILCs), which are somewhat similar to T cells in their cellular features but do not have receptors that specifically recognize antigens. ILCs are present deep in tissues such as lungs, intestine, and skin, and play crucial roles in the maintenance and defense of peripheral tissues.
According to Kim, "A major problem is that these cells undergo natural and inflammation-induced attrition over time, leading to ILC insufficiency, weakened barrier function, and increased infection. A potential solution for this problem would be increased mobilization of bone marrow (BM) ILC progenitors to make up the natural loss of tissue ILCs," but the mechanisms for this were unclear.
The Kim laboratory recently discovered that the progenitors for ILCs (ILCPs) are divided into two subsets in distinct niches of the BM (i.e., sinusoid and parenchyma). Sinusoid ILCPs emigrate from the BM to circulate in the peripheral blood and join various peripheral tissues to make mature ILCs.
In contrast, parenchyma ILCPs undergo maturation and population expansion within the BM to potentially maintain the central ILC reservoir. The mobilization of BM ILCPs is controlled by the coordinated expression of the BM retention receptors (Itg-α4 and CXCR4) and the emigration receptors sphingosine-1-phosphate (S1P) receptors.
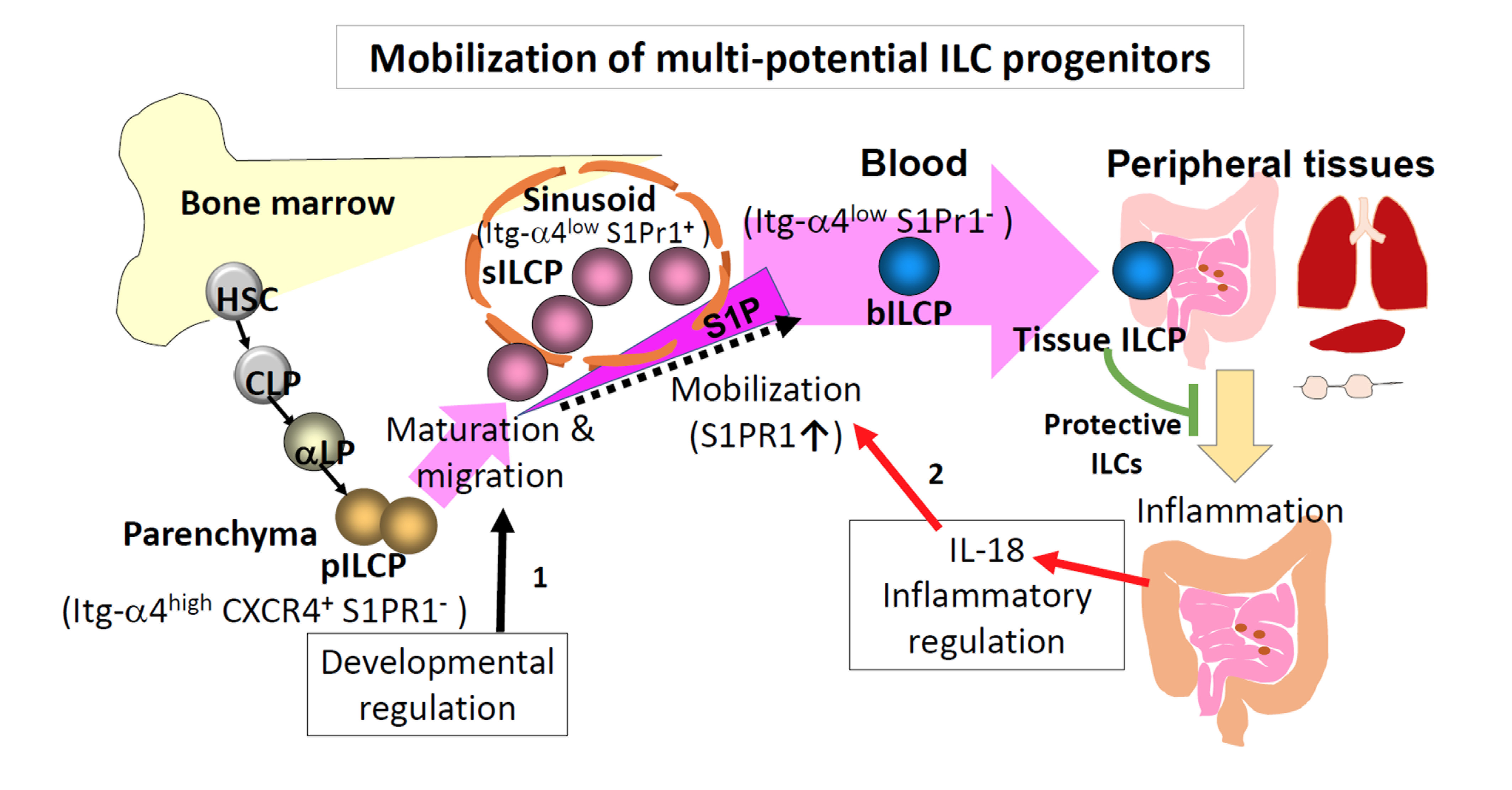
Importantly, Kim’s lab also discovered that the inflammatory cytokine IL-18 induces the mobilization of ILCPs from the BM. Mobilized BM ILCPs can effectively protect tissues during inflammatory responses. "These findings demonstrate how the ILC pool in the body depends in part on the bone marrow-emigrating subset of ILCPs," explained Kim. This important discovery provides new research opportunities for possible therapeutic utilization of these ILCPs to restore innate immunity and protect barrier tissues in pathological conditions.
Citation: Liu Q, Lee JH, Kang HM, Kim CH. Identification of the niche and mobilization mechanism for tissue-protective multipotential bone marrow ILC progenitors. Sci Adv. 2022 Nov 25;8(47):eabq1551. doi: 10.1126/sciadv.abq1551. Epub 2022 Nov 23. PubMed PMID: 36417511; PubMed Central PMCID: PMC9683709.
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
 ON THE COVER
ON THE COVER
